Back
Poster Session D
Session: (2052–2107) SLE – Diagnosis, Manifestations, and Outcomes Poster III: Outcomes
2062: Predicting Severe Flares: Real-world Application of a Decision-Tree Model Among the Medicaid-Insured Systemic Lupus Erythematosus (SLE) Population in the US
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SW
Sandra Wu, PhD
AstraZeneca
Hockessin, DE, United States
Abstract Poster Presenter(s)
Sandra Sze-jung Wu1, Allison Perry2, Nicole Zimmerman3, Helen Varker4, Rich Bizier4, Liisa Palmer4, Christine Dube5 and Gary Bryant6, 1AstraZeneca, Hockessin, DE, 2IBM Watson Health, New York, NY, 3IBM Watson Health, Chagrin Falls, OH, 4Merative, Cambridge, MA, 5AstraZeneca, Torrington, CT, 6AstraZeneca, New Castle, DE
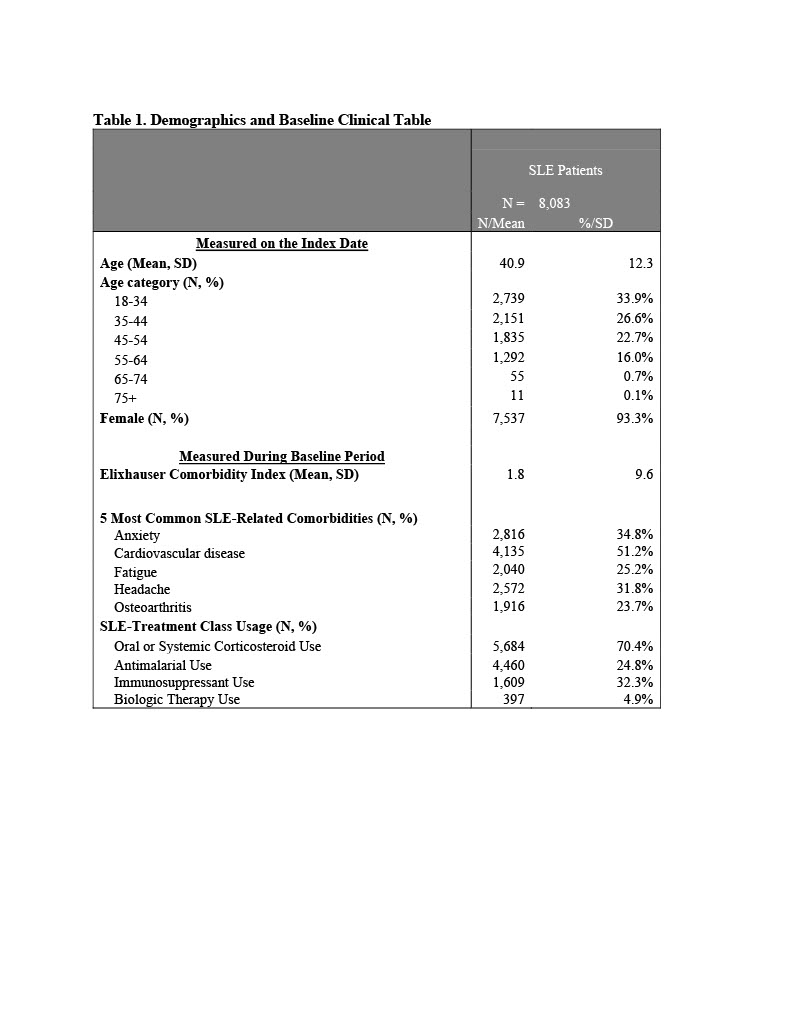
Background/Purpose: Systemic lupus erythematosus (SLE) is a complex disease with multisystem inflammation resulting in variable clinical manifestations. As a result, predicting the occurrence of an SLE flare among patients remains a challenge for physicians.
To identify risk factors associated with SLE flares requiring an emergency room visit (ER) or inpatient (IP) admission, among Medicaid enrollees with prevalent SLE using an easy-to-implement predictive decision tree model.
Methods: Patients with SLE were selected from IBM MarketScan Multi-State Medicaid Database (2013-2019) based on ≥1 inpatient claim with a SLE diagnosis, or ≥2 non-diagnostic SLE outpatient claims. A prevalent cohort was created by randomly selecting an index date ≥12 months following first SLE claim. Also required: continuous medical and pharmacy benefits for 12 months pre-index (baseline) and post-index (Year 2) and valid steroid prescription claims. SLE flares were defined using published algorithm based on presence of SLE-related treatment and diagnoses.1 A classification and regression tree (CART) model was constructed to examine combinations of factors associated with developing SLE flares. Data were randomly split into training (75%) and validation (25%) datasets. At each node, the tree was split on the predictor and split value that minimized the Gini impurity. Predictors included demographic characteristics as well as baseline comorbid conditions, medication use, ER visits, and IP admissions. Splitting continued until maximum tree depth, and then pruned by penalizing the purity criterion. The optimal complexity parameter was selected using 10-fold cross validation repeated 10 times. The validation dataset was used to evaluate the tree's predictive performance based on the area under the receiver operator curve (ROC) and the Brier score. The predicted probability (Pr) of having an ER/IP flare was computed for each patient in the validation dataset.
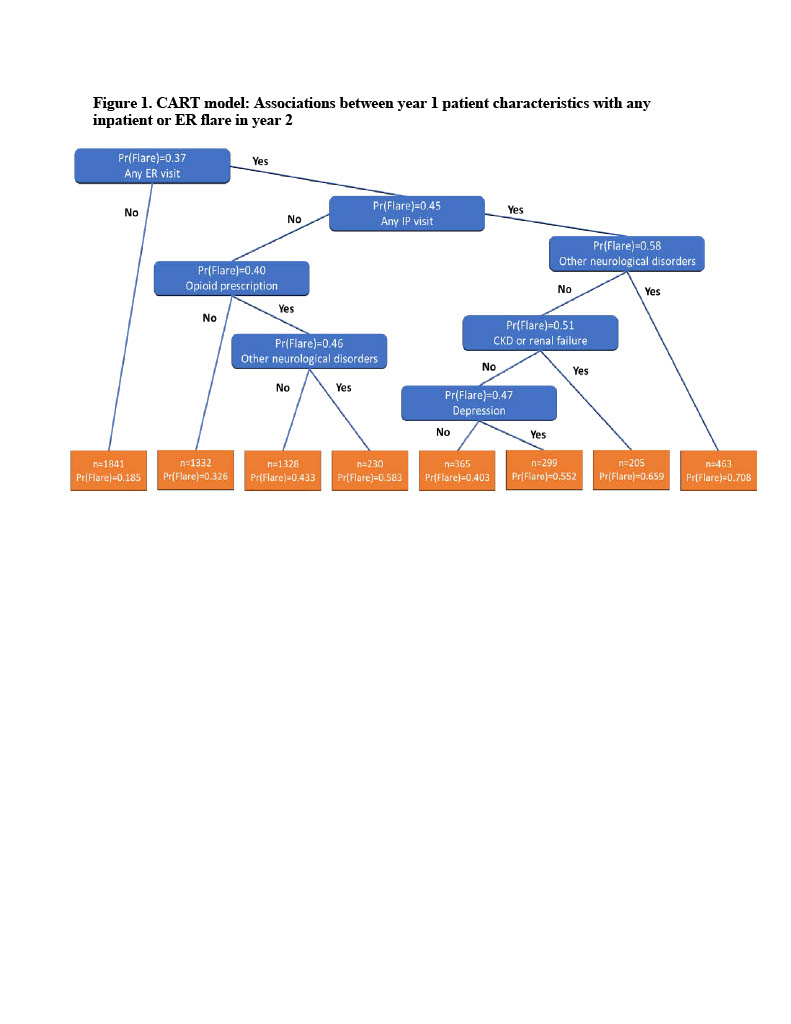
Results: Patients without a baseline ER visit had the lowest probability of a year-2 inpatient or ER flare (Pr(Flare)=0.185), while patients with a baseline ER visit, a baseline IP admission, and evidence of non-paralysis neurological disorders had the highest probability of an IP or ER flare in year 2 (Pr(Flare)=0.708). Presence of opioid prescriptions, chronic kidney disease or renal failure, and depression also increase the probability of a future inpatient or ER flare. The C-statistic for the selected covariates was 0.72. Based on the CART model, the leading patient characteristics related to any IP or ER flare in year 2 included opioids (variable importance = 1.00), other neurological disorders (0.96), any baseline ER visit (0.92), any inpatient admission (0.89), depression (0.64), and chronic kidney disease or renal failure (0.32).
Conclusion: The model effectively identified subgroups of Medicaid insured SLE patients with particularly high probabilities for an SLE flare. In addition, the model identified that resorting to opioids for pain management was the most important individual predictor of an SLE flare, and therefore an important marker of Medicaid patients in need of more effective SLE disease management.
1. Garris C, et al. J Med Econ. 2013;16(5):667-677.
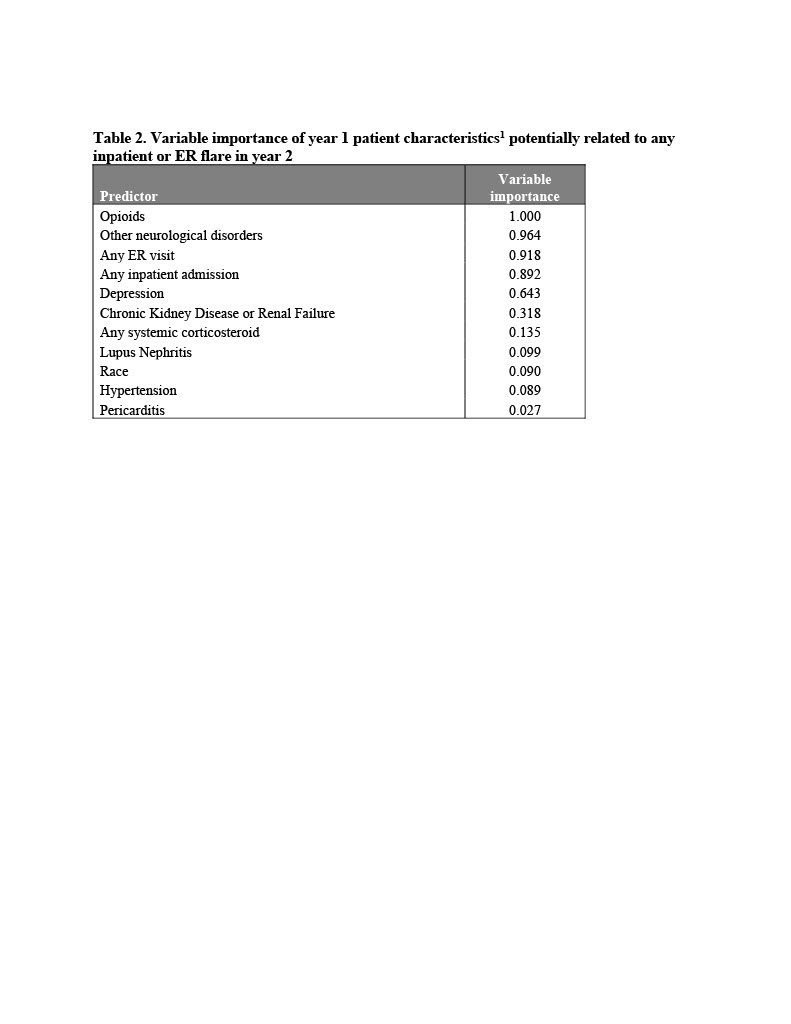 The following characteristics were also included in the model, but had a variable importance of 0.000: Age ≥ 45, Age ≥ 65, Sex, Insurance Plan Type, Cancer, Chronic pulmonary disease, Coagulopathy, Congestive heart failure, Diabetes, Drug abuse, Liver disease, Peripheral vascular disorders, Anxiety, Atherosclerosis, Cerebrovascular disease, stroke, or TIA, Endocarditis, Myocardial infarction, Fibromyalgia, Fractures, Kidney Transplant, Osteoarthritis, Osteoporosis, Pleurisy/Pleural Effusion, Raynaud's Disease, Thrombocytopenia, Antidepressants, Antihypertensives
The following characteristics were also included in the model, but had a variable importance of 0.000: Age ≥ 45, Age ≥ 65, Sex, Insurance Plan Type, Cancer, Chronic pulmonary disease, Coagulopathy, Congestive heart failure, Diabetes, Drug abuse, Liver disease, Peripheral vascular disorders, Anxiety, Atherosclerosis, Cerebrovascular disease, stroke, or TIA, Endocarditis, Myocardial infarction, Fibromyalgia, Fractures, Kidney Transplant, Osteoarthritis, Osteoporosis, Pleurisy/Pleural Effusion, Raynaud's Disease, Thrombocytopenia, Antidepressants, Antihypertensives
Disclosures: S. Wu, AstraZeneca; A. Perry, None; N. Zimmerman, IBM Watson Health, GlaxoSmithKlein(GSK), AstraZeneca; H. Varker, None; R. Bizier, None; L. Palmer, None; C. Dube, AstraZeneca; G. Bryant, AstraZeneca, AstraZeneca.
Background/Purpose: Systemic lupus erythematosus (SLE) is a complex disease with multisystem inflammation resulting in variable clinical manifestations. As a result, predicting the occurrence of an SLE flare among patients remains a challenge for physicians.
To identify risk factors associated with SLE flares requiring an emergency room visit (ER) or inpatient (IP) admission, among Medicaid enrollees with prevalent SLE using an easy-to-implement predictive decision tree model.
Methods: Patients with SLE were selected from IBM MarketScan Multi-State Medicaid Database (2013-2019) based on ≥1 inpatient claim with a SLE diagnosis, or ≥2 non-diagnostic SLE outpatient claims. A prevalent cohort was created by randomly selecting an index date ≥12 months following first SLE claim. Also required: continuous medical and pharmacy benefits for 12 months pre-index (baseline) and post-index (Year 2) and valid steroid prescription claims. SLE flares were defined using published algorithm based on presence of SLE-related treatment and diagnoses.1 A classification and regression tree (CART) model was constructed to examine combinations of factors associated with developing SLE flares. Data were randomly split into training (75%) and validation (25%) datasets. At each node, the tree was split on the predictor and split value that minimized the Gini impurity. Predictors included demographic characteristics as well as baseline comorbid conditions, medication use, ER visits, and IP admissions. Splitting continued until maximum tree depth, and then pruned by penalizing the purity criterion. The optimal complexity parameter was selected using 10-fold cross validation repeated 10 times. The validation dataset was used to evaluate the tree's predictive performance based on the area under the receiver operator curve (ROC) and the Brier score. The predicted probability (Pr) of having an ER/IP flare was computed for each patient in the validation dataset.
Results: Patients without a baseline ER visit had the lowest probability of a year-2 inpatient or ER flare (Pr(Flare)=0.185), while patients with a baseline ER visit, a baseline IP admission, and evidence of non-paralysis neurological disorders had the highest probability of an IP or ER flare in year 2 (Pr(Flare)=0.708). Presence of opioid prescriptions, chronic kidney disease or renal failure, and depression also increase the probability of a future inpatient or ER flare. The C-statistic for the selected covariates was 0.72. Based on the CART model, the leading patient characteristics related to any IP or ER flare in year 2 included opioids (variable importance = 1.00), other neurological disorders (0.96), any baseline ER visit (0.92), any inpatient admission (0.89), depression (0.64), and chronic kidney disease or renal failure (0.32).
Conclusion: The model effectively identified subgroups of Medicaid insured SLE patients with particularly high probabilities for an SLE flare. In addition, the model identified that resorting to opioids for pain management was the most important individual predictor of an SLE flare, and therefore an important marker of Medicaid patients in need of more effective SLE disease management.
1. Garris C, et al. J Med Econ. 2013;16(5):667-677.


 The following characteristics were also included in the model, but had a variable importance of 0.000: Age ≥ 45, Age ≥ 65, Sex, Insurance Plan Type, Cancer, Chronic pulmonary disease, Coagulopathy, Congestive heart failure, Diabetes, Drug abuse, Liver disease, Peripheral vascular disorders, Anxiety, Atherosclerosis, Cerebrovascular disease, stroke, or TIA, Endocarditis, Myocardial infarction, Fibromyalgia, Fractures, Kidney Transplant, Osteoarthritis, Osteoporosis, Pleurisy/Pleural Effusion, Raynaud's Disease, Thrombocytopenia, Antidepressants, Antihypertensives
The following characteristics were also included in the model, but had a variable importance of 0.000: Age ≥ 45, Age ≥ 65, Sex, Insurance Plan Type, Cancer, Chronic pulmonary disease, Coagulopathy, Congestive heart failure, Diabetes, Drug abuse, Liver disease, Peripheral vascular disorders, Anxiety, Atherosclerosis, Cerebrovascular disease, stroke, or TIA, Endocarditis, Myocardial infarction, Fibromyalgia, Fractures, Kidney Transplant, Osteoarthritis, Osteoporosis, Pleurisy/Pleural Effusion, Raynaud's Disease, Thrombocytopenia, Antidepressants, AntihypertensivesDisclosures: S. Wu, AstraZeneca; A. Perry, None; N. Zimmerman, IBM Watson Health, GlaxoSmithKlein(GSK), AstraZeneca; H. Varker, None; R. Bizier, None; L. Palmer, None; C. Dube, AstraZeneca; G. Bryant, AstraZeneca, AstraZeneca.

