Back
Poster Session B
Antiphospholipid Syndrome
Session: (0671–0694) Antiphospholipid Syndrome Poster
0688: Antibodies of the IgA Isotype Target Neutrophil Extracellular Traps in APS
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- SN
Sherwin Navaz, BS
University of Michigan
Ann Arbor, MI, United States
Abstract Poster Presenter(s)
Sherwin Navaz1, Lyndsay Kluge1, Katarina Kmetova2, Bruna Jacintho3, Srilakshmi Yalavarthi1, Claire Hoy1, Cyrus Sarosh4, Noah Peters1, Tristin Smith1, Gabriel Figueroa Parra5, Jacqueline Madison1, Kristen Demoruelle6, Ali Duarte-Garcia5, Jason S Knight7 and Yu (Ray) Zuo1, 1University of Michigan, Ann Arbor, MI, 2Division of Rheumatology, University of Michigan, Ann Arbor, MI, 3University of Michigan, São Paulo, Brazil, 4University of Michigan, Temperance, MI, 5Mayo Clinic, Rochester, MN, 6University of Colorado Anschutz Medical Campus, Aurora, CO, 7University of Michigan, Division of Rheumatology, Ann Arbor, MI
Background/Purpose: Neutrophil extracellular traps (NETs) are prothrombotic and proinflammatory webs of nuclear DNA, histones, and microbicidal proteins extruded by neutrophils into the extracellular space in order to trap and kill pathogens. Overexuberant release of NETs by antiphospholipid antibody (aPL)-activated neutrophils contributes to APS pathogenesis. Recent work has demonstrated that some patients with APS develop autoantibodies targeting NETs (anti-NET IgG and IgM), which impair NET clearance and activate complement. Here, we aimed to evaluate the presence and potential clinical associations of anti-NET IgA isotype antibodies in a large cohort of APS patients.
Methods: Levels of anti-NET IgA, IgG, and IgM were measured by a novel ELISA platform in the plasma of 111 patients with primary APS, 34 patients with secondary APS, 41 patients who were persistently aPL-positive but without "criteria" APS manifestations, and 42 healthy controls (Fig 1A). Some criteria aPL (anticardiolipin IgG/M; and anti-beta-2 glycoprotein I IgG/M) and non-criteria aPL (anticardiolipin IgA; anti-beta-2 glycoprotein I IgA; and anti-phosphatidylserine/prothrombin IgG/M) were quantified by QUANTA Lite ELISAs (Werfen). Circulating NET remnants were assessed by ELISA. Statistical analysis, including the determination of correlations by Spearman's method, was performed using GraphPad Prism 8.0.
Results: Elevated levels of anti-NET IgA were detected in many patients with primary and secondary APS, as well as in aPL-positive patients who lacked criteria clinical manifestations (Fig 1B). There was a positive correlation between anti-NET IgA and IgG (r=0.35, p< 0.0001) but not between anti-NET IgA and IgM (r=0.14, p=0.06). High anti-NET IgA levels were also associated with biomarkers of NET remnants as measured by myeloperoxidase-DNA complexes (r=0.26, p=0.005) and calprotectin (r=0.20, p=0.01). Interestingly, anti-NET IgA also demonstrated a positive correlation with IgA isotypes of traditional aPL (anticardiolipin IgA: r=0.29, p< 0.0001; and anti-beta-2 glycoprotein I IgA: r=0.42, p< 0.0001), but not with the IgG or IgM isotypes of any of the tested aPL. Clinically, anti-NET IgA tracked with increased anti-double-stranded DNA antibodies (r=0.41, p< 0.0001), elevated CRP (r=0.22, p=0.03), and renal impairment as defined by higher serum creatinine (r=0.20, p=0.02).
Conclusion: In summary, these data reveal high levels of anti-NET IgA in many individuals with persistent aPL positivity, whether they meet clinical criteria for APS or not. Moreover, anti-NET IgA tracks with circulating markers of NETs, increased inflammation, and impaired renal function. Studies are underway to define the potential mechanistic roles of anti-NET IgA in the APS thromboinflammatory milieu.
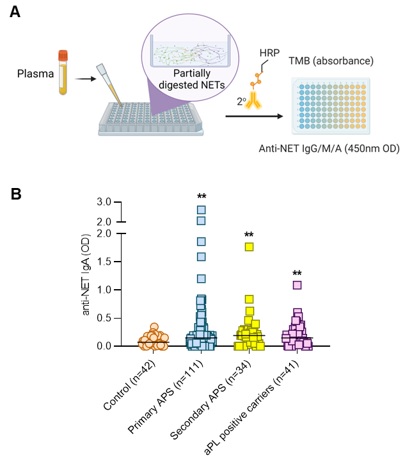 Figure 1: High anti-NET IgA in some individuals with persistently positive aPL. A, Schematic illustration of anti-NET ELISA. Unique secondary antibodies are used to probe for different isotypes. B, Levels of anti-NET IgA were measured in the indicated groups and compared with healthy controls by Kruskal-Wallis test corrected for multiple comparisons by Dunn’s method; **p < 0.01.
Figure 1: High anti-NET IgA in some individuals with persistently positive aPL. A, Schematic illustration of anti-NET ELISA. Unique secondary antibodies are used to probe for different isotypes. B, Levels of anti-NET IgA were measured in the indicated groups and compared with healthy controls by Kruskal-Wallis test corrected for multiple comparisons by Dunn’s method; **p < 0.01.
Disclosures: S. Navaz, None; L. Kluge, None; K. Kmetova, None; B. Jacintho, None; S. Yalavarthi, None; C. Hoy, None; C. Sarosh, None; N. Peters, None; T. Smith, None; G. Figueroa Parra, None; J. Madison, None; K. Demoruelle, Boehringer-Ingelheim, Pfizer; A. Duarte-Garcia, None; J. Knight, Jazz Pharmaceuticals, Bristol Myers Squibb; Y. Zuo, None.
Background/Purpose: Neutrophil extracellular traps (NETs) are prothrombotic and proinflammatory webs of nuclear DNA, histones, and microbicidal proteins extruded by neutrophils into the extracellular space in order to trap and kill pathogens. Overexuberant release of NETs by antiphospholipid antibody (aPL)-activated neutrophils contributes to APS pathogenesis. Recent work has demonstrated that some patients with APS develop autoantibodies targeting NETs (anti-NET IgG and IgM), which impair NET clearance and activate complement. Here, we aimed to evaluate the presence and potential clinical associations of anti-NET IgA isotype antibodies in a large cohort of APS patients.
Methods: Levels of anti-NET IgA, IgG, and IgM were measured by a novel ELISA platform in the plasma of 111 patients with primary APS, 34 patients with secondary APS, 41 patients who were persistently aPL-positive but without "criteria" APS manifestations, and 42 healthy controls (Fig 1A). Some criteria aPL (anticardiolipin IgG/M; and anti-beta-2 glycoprotein I IgG/M) and non-criteria aPL (anticardiolipin IgA; anti-beta-2 glycoprotein I IgA; and anti-phosphatidylserine/prothrombin IgG/M) were quantified by QUANTA Lite ELISAs (Werfen). Circulating NET remnants were assessed by ELISA. Statistical analysis, including the determination of correlations by Spearman's method, was performed using GraphPad Prism 8.0.
Results: Elevated levels of anti-NET IgA were detected in many patients with primary and secondary APS, as well as in aPL-positive patients who lacked criteria clinical manifestations (Fig 1B). There was a positive correlation between anti-NET IgA and IgG (r=0.35, p< 0.0001) but not between anti-NET IgA and IgM (r=0.14, p=0.06). High anti-NET IgA levels were also associated with biomarkers of NET remnants as measured by myeloperoxidase-DNA complexes (r=0.26, p=0.005) and calprotectin (r=0.20, p=0.01). Interestingly, anti-NET IgA also demonstrated a positive correlation with IgA isotypes of traditional aPL (anticardiolipin IgA: r=0.29, p< 0.0001; and anti-beta-2 glycoprotein I IgA: r=0.42, p< 0.0001), but not with the IgG or IgM isotypes of any of the tested aPL. Clinically, anti-NET IgA tracked with increased anti-double-stranded DNA antibodies (r=0.41, p< 0.0001), elevated CRP (r=0.22, p=0.03), and renal impairment as defined by higher serum creatinine (r=0.20, p=0.02).
Conclusion: In summary, these data reveal high levels of anti-NET IgA in many individuals with persistent aPL positivity, whether they meet clinical criteria for APS or not. Moreover, anti-NET IgA tracks with circulating markers of NETs, increased inflammation, and impaired renal function. Studies are underway to define the potential mechanistic roles of anti-NET IgA in the APS thromboinflammatory milieu.
 Figure 1: High anti-NET IgA in some individuals with persistently positive aPL. A, Schematic illustration of anti-NET ELISA. Unique secondary antibodies are used to probe for different isotypes. B, Levels of anti-NET IgA were measured in the indicated groups and compared with healthy controls by Kruskal-Wallis test corrected for multiple comparisons by Dunn’s method; **p < 0.01.
Figure 1: High anti-NET IgA in some individuals with persistently positive aPL. A, Schematic illustration of anti-NET ELISA. Unique secondary antibodies are used to probe for different isotypes. B, Levels of anti-NET IgA were measured in the indicated groups and compared with healthy controls by Kruskal-Wallis test corrected for multiple comparisons by Dunn’s method; **p < 0.01.Disclosures: S. Navaz, None; L. Kluge, None; K. Kmetova, None; B. Jacintho, None; S. Yalavarthi, None; C. Hoy, None; C. Sarosh, None; N. Peters, None; T. Smith, None; G. Figueroa Parra, None; J. Madison, None; K. Demoruelle, Boehringer-Ingelheim, Pfizer; A. Duarte-Garcia, None; J. Knight, Jazz Pharmaceuticals, Bristol Myers Squibb; Y. Zuo, None.

