Back
Poster Session D
Session: (2108–2153) Spondyloarthritis Including PsA – Treatment Poster III: PsA
2138: Real-world Persistence and Treatment Patterns in Psoriatic Arthritis Patients Treated with Anti-IL17 Therapy
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SM
Sebastian Moyano, MD
Lilly
Alcobendas, Madrid, Spain
Abstract Poster Presenter(s)
BEATRIZ E. JOVEN1, Concepción Fito Manteca2, Enrique Rubio3, Enrique Raya Álvarez4, Alba Pérez5, Raquel Hernández6, Sara Manrique Arija7, Mercedes Núñez8, Silvia Díaz8, Luis Trancho8, Sebastian Moyano8, Alessandra Lacetera9, Noelia Alfaro-Oliver9 and Rosario Garcia-Vicuña10, 1Hospital Universitario 12 de Octubre, Madrid, Spain, 2Hospitalario de Navarra, Pamplona, Spain, 3Division of Rheumatology, Hospital Universitario Virgen del Rocío, Sevilla, Spain, 4Hospital Universitario San Cecilio, Granada, Spain, 5Hospital Puerta del Mar, Cádiz, Spain, 6Virgen de Valme University Hospital, Sevilla, Spain, 7Hospital Regional Universitario Málaga, Málaga, Spain, 8Lilly, Alcobendas, Spain, 9OXON Epidemiology, Madrid, Spain, 10Hospital Universitario de La Princesa, IIS-Princesa, Madrid, Spain
Background/Purpose: Interleukin-17A inhibitors (anti-IL17) have provided an additional treatment option in the management of the psoriatic arthritis (PsA). This study aims to describe the patient profile, treatment patterns and persistence in PsA patients treated with ixekizumab and secukinumab in a real-life setting.
Methods: A multicenter retrospective study was conducted at 8 Spanish hospitals. Three cohorts of adult PsA patients, newly initiating treatment with an anti-IL17 (secukinumab 150mg [SECU150], secukinumab 300mg [SECU300] or ixekizumab [IXE]), between January-2019 and March-2020 were included. Data of patients exposed to anti-IL17 drugs and with a follow-up visit available were collected until March-2021. Demographic and clinical characteristics, treatment patterns, and persistence were analyzed descriptively. Continuous data were presented as mean (Standard Deviation (SD)) and categorical variables as frequencies with percentage. Persistence rates at 3/6/12 months were calculated.
Results: A total of 221 PsA patients were analysed (SECU150: 103 [46.6%], SECU300: 38 [17.2%] and IXE: 80 [36.2%]). Patients in the SECU150 group presented higher proportion of moderate PsA and less peripheral joint counts. The highest proportion of patients with enthesitis and active skin lessions due to psoriasis in the moment of the prescription was in the SECU300 group. Patients in the IXE group showed longer time since PsA diagnosis, had more frequent comorbidities, joint damage and psoriasis diagnosed (Table 1). 77.8% of patients were previously treated with csDMARDs in monotherapy and 72.9% with bDMARDs/tsDMARDs. The lowest proportion of patients with a prior bDMARDS/tsDMARDS use was observed in the SECU150 group (58.3%), followed by SECU300 (68.4%) and the highest in the IXE group (93.8%). Mean number of previous bDMARDS/tsDMARDS for SECU150, SECU300 and IXE groups were 1.6(1.0), 1.7 (0.9) and 2.4 (1.5), respectively. Anti-IL17 persistence is shown in Figure 1. Most frequent reason for discontinuation was lack of effectiveness (13.8%).
Conclusion: Most of PsA patients treated with anti-IL17 in Spain had a moderate or high disease activity, high peripheral joint damage and skin involvement and had received at least 1 previous bDMARDS/tsDMARDS. More than 80% of patients with one year follow-up were persistent to anti-IL17 treatments, observing the highest rate with IXE, followed by SECU150 and SECU300.
.jpg) Table 1- Demographic and clinical patient characteristics at baseline
Table 1- Demographic and clinical patient characteristics at baseline
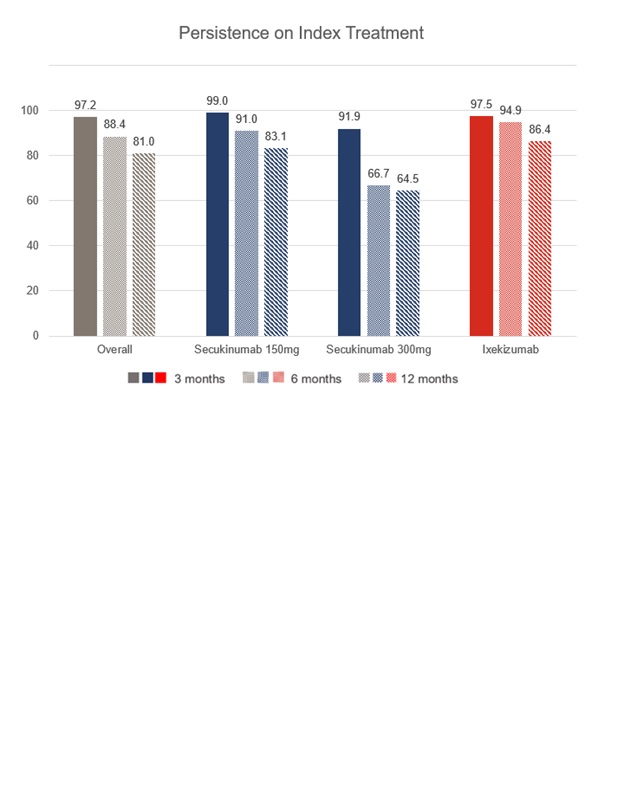 Figure 1- Anti-IL17 persistence, overall and by study cohorts over one year follow-up
Figure 1- Anti-IL17 persistence, overall and by study cohorts over one year follow-up
Disclosures: B. JOVEN, Novartis, UCB, Janssen, Amgen, AbbVie/Abbott, Eli Lilly; C. Fito Manteca, None; E. Rubio, None; E. Raya Álvarez, None; A. Pérez, None; R. Hernández, Novartis, Janssen, Pfizer, AbbVie/Abbott, Eli Lilly; S. Manrique Arija, None; M. Núñez, Eli Lilly; S. Díaz, Eli Lilly; L. Trancho, Eli Lilly; S. Moyano, Eli Lilly; A. Lacetera, None; N. Alfaro-Oliver, None; R. Garcia-Vicuña, Novartis, Sanofi, Merck/MSD, Bristol-Myers Squibb(BMS), Eli Lilly, UCB, Janssen, Sandoz, Pfizer, Biogen.
Background/Purpose: Interleukin-17A inhibitors (anti-IL17) have provided an additional treatment option in the management of the psoriatic arthritis (PsA). This study aims to describe the patient profile, treatment patterns and persistence in PsA patients treated with ixekizumab and secukinumab in a real-life setting.
Methods: A multicenter retrospective study was conducted at 8 Spanish hospitals. Three cohorts of adult PsA patients, newly initiating treatment with an anti-IL17 (secukinumab 150mg [SECU150], secukinumab 300mg [SECU300] or ixekizumab [IXE]), between January-2019 and March-2020 were included. Data of patients exposed to anti-IL17 drugs and with a follow-up visit available were collected until March-2021. Demographic and clinical characteristics, treatment patterns, and persistence were analyzed descriptively. Continuous data were presented as mean (Standard Deviation (SD)) and categorical variables as frequencies with percentage. Persistence rates at 3/6/12 months were calculated.
Results: A total of 221 PsA patients were analysed (SECU150: 103 [46.6%], SECU300: 38 [17.2%] and IXE: 80 [36.2%]). Patients in the SECU150 group presented higher proportion of moderate PsA and less peripheral joint counts. The highest proportion of patients with enthesitis and active skin lessions due to psoriasis in the moment of the prescription was in the SECU300 group. Patients in the IXE group showed longer time since PsA diagnosis, had more frequent comorbidities, joint damage and psoriasis diagnosed (Table 1). 77.8% of patients were previously treated with csDMARDs in monotherapy and 72.9% with bDMARDs/tsDMARDs. The lowest proportion of patients with a prior bDMARDS/tsDMARDS use was observed in the SECU150 group (58.3%), followed by SECU300 (68.4%) and the highest in the IXE group (93.8%). Mean number of previous bDMARDS/tsDMARDS for SECU150, SECU300 and IXE groups were 1.6(1.0), 1.7 (0.9) and 2.4 (1.5), respectively. Anti-IL17 persistence is shown in Figure 1. Most frequent reason for discontinuation was lack of effectiveness (13.8%).
Conclusion: Most of PsA patients treated with anti-IL17 in Spain had a moderate or high disease activity, high peripheral joint damage and skin involvement and had received at least 1 previous bDMARDS/tsDMARDS. More than 80% of patients with one year follow-up were persistent to anti-IL17 treatments, observing the highest rate with IXE, followed by SECU150 and SECU300.
.jpg) Table 1- Demographic and clinical patient characteristics at baseline
Table 1- Demographic and clinical patient characteristics at baseline Figure 1- Anti-IL17 persistence, overall and by study cohorts over one year follow-up
Figure 1- Anti-IL17 persistence, overall and by study cohorts over one year follow-upDisclosures: B. JOVEN, Novartis, UCB, Janssen, Amgen, AbbVie/Abbott, Eli Lilly; C. Fito Manteca, None; E. Rubio, None; E. Raya Álvarez, None; A. Pérez, None; R. Hernández, Novartis, Janssen, Pfizer, AbbVie/Abbott, Eli Lilly; S. Manrique Arija, None; M. Núñez, Eli Lilly; S. Díaz, Eli Lilly; L. Trancho, Eli Lilly; S. Moyano, Eli Lilly; A. Lacetera, None; N. Alfaro-Oliver, None; R. Garcia-Vicuña, Novartis, Sanofi, Merck/MSD, Bristol-Myers Squibb(BMS), Eli Lilly, UCB, Janssen, Sandoz, Pfizer, Biogen.

