Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0883–0912) RA – Diagnosis, Manifestations, and Outcomes Poster II
0885: Association Between Changes in Inflammatory Pathways Associated with a Reduction in CV Risk: Results from the LiiRA Study
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- bw
brittany weber, MD, PhD
Brigham and Women's Hospital
DEDHAM, MA, United States
Abstract Poster Presenter(s)
brittany weber1, Dana Weisenfeld1, Thany Seyok1, Elena Massarotti1, Selena Huang1, Derrick Todd1, Jonathan Coblyn1, michael weinblatt1, Tianrun Cai1, Kumar Dahal1, leanne BArrett1, Daniel Solomon1, Jorge Plutzky1, Marcy Bolster2, marcelo Dicarli1 and Katherine Liao1, 1Brigham and Women's Hospital, Boston, MA, 2Massachusetts General Hospital, Boston, MA
Background/Purpose: Patients with rheumatoid arthritis (RA) are at increased cardiovascular (CV) risk. Coronary flow reserve (CFR) is a quantitative imaging biomarker of CV risk that can detect coronary microvascular disease (CMD), associated with higher mortality in RA. Additionally, subclinical myocardial injury can be assessed with high sensitivity cardiac troponin (hs-cTnT). The objective of this study was to determine the association between a reduction in inflammation with changes in CV risk as measured by CFR and hs-cTnT, as part of the LiiRA study.
Methods: The Lipids, Inflammation and CV risk in RA (LiiRA) study was a case-crossover study, recruiting RA patients age ≥18 years with active disease about to initiate a TNFi as part of clinical care. Exclusion criteria include prior CVD, statin use, prednisone dose >10mg, or prior biologic DMARD use. All subjects underwent a stress myocardial perfusion PET scan (cardiac PET) at baseline and after 24 weeks. Coronary flow reserve (CFR) is calculated from cardiac PET data using a ratio of myocardial blood flow (MBF) during maximal hyperemia over that at rest. A lower CFR in the absence of obstructive coronary artery disease (CAD) is indicative of CMD, defined as CFR< 2.5. Blood measurements for routine lipids, inflammatory markers including hsCRP, IL-6, IL-1beta, and hs-cTnT were also performed at baseline and follow-up. The primary outcome was change in CFR at baseline and 24 weeks with a secondary outcome of change in hs-cTnT. We tested the correlations between change in inflammation with change in the primary and secondary outcomes using Spearman's correlations.
Results: In total, n=66 subjects completed the protocol with the primary outcome; 80% female, mean RA duration 7.7 years, 69% seropositive. The mean estimated baseline 10-year estimated ASCVD risk was 5.6%, yet 47% had evidence of CMD. We observed a significant reduction in all inflammatory markers between baseline and follow-up, as well as improvements in RA disease activity (Table 1); however, we did not observe a significant change in CFR and hs-cTnT . No correlations were observed between change in inflammatory marker with change in CFR. A reduction in hsCRP and IL-1beta was correlated with a reduction in hs-cTnT (Table 2). A non-significant trend for correlation of reduction of IL-1beta with higher CFR was observed among subjects with evidence of CMD at baseline (Table 3).
Conclusion: In one of the largest cohorts of RA subjects with detailed data on inflammatory markers, subclinical markers of myocardial injury, and CFR, reducing inflammation was correlated with improvements in measures of CV risk in a subset of patients with evidence of subclinical myocardial injury and potentially CMD via the IL-1beta pathway. In this population of overall low/borderline ASCVD risk, blood and imaging markers may inform which RA patients may benefit from CV risk reduction by targeting inflammation.
.jpg) Table 1. Change in inflammatory markers, disease activity, and measure of CV risk at baseline and follow-up, all values shown in median (interquartile range).
Table 1. Change in inflammatory markers, disease activity, and measure of CV risk at baseline and follow-up, all values shown in median (interquartile range).
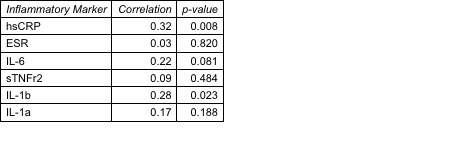 Table 2. Correlation between change in inflammatory markers with change in hs-cTnT.
Table 2. Correlation between change in inflammatory markers with change in hs-cTnT.
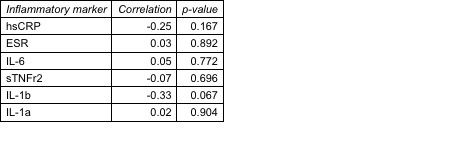 Table 3. Correlation between change in inflammatory markers with change in CFR, among subjects with CMD, n=31 (CFR < 2.5).
Table 3. Correlation between change in inflammatory markers with change in CFR, among subjects with CMD, n=31 (CFR < 2.5).
Disclosures: b. weber, Horizon Therapeutics; D. Weisenfeld, None; T. Seyok, None; E. Massarotti, None; S. Huang, None; D. Todd, None; J. Coblyn, None; m. weinblatt, AbbVie/Abbott, Acclaris, Bristol Myers Squibb, Corevitas, egrx, Genosco, Horizon Therapeutics, GlaxoSmithKlein(GSK), Gilead, johnson and johnson, Eli Lilly, Pfizer, Roche, Scipher, Sanofi, set point, Tremeau, Bristol Myers Squibb, sanofi, Amgen, Eli Lilly, Canfite, Inmedix, Scipher; T. Cai, None; K. Dahal, None; l. BArrett, None; D. Solomon, AbbVie/Abbott, Amgen, moderna, CorEvitas; J. Plutzky, Amgen, Boehringer-Ingelheim, Novo Nordisk; M. Bolster, Johnson and Johnson, Merck Manual, Cumberland, Corbus Inc., PracticeUpdate, Rheumatology Research Foundation; m. Dicarli, Gilead Sciences; K. Liao, None.
Background/Purpose: Patients with rheumatoid arthritis (RA) are at increased cardiovascular (CV) risk. Coronary flow reserve (CFR) is a quantitative imaging biomarker of CV risk that can detect coronary microvascular disease (CMD), associated with higher mortality in RA. Additionally, subclinical myocardial injury can be assessed with high sensitivity cardiac troponin (hs-cTnT). The objective of this study was to determine the association between a reduction in inflammation with changes in CV risk as measured by CFR and hs-cTnT, as part of the LiiRA study.
Methods: The Lipids, Inflammation and CV risk in RA (LiiRA) study was a case-crossover study, recruiting RA patients age ≥18 years with active disease about to initiate a TNFi as part of clinical care. Exclusion criteria include prior CVD, statin use, prednisone dose >10mg, or prior biologic DMARD use. All subjects underwent a stress myocardial perfusion PET scan (cardiac PET) at baseline and after 24 weeks. Coronary flow reserve (CFR) is calculated from cardiac PET data using a ratio of myocardial blood flow (MBF) during maximal hyperemia over that at rest. A lower CFR in the absence of obstructive coronary artery disease (CAD) is indicative of CMD, defined as CFR< 2.5. Blood measurements for routine lipids, inflammatory markers including hsCRP, IL-6, IL-1beta, and hs-cTnT were also performed at baseline and follow-up. The primary outcome was change in CFR at baseline and 24 weeks with a secondary outcome of change in hs-cTnT. We tested the correlations between change in inflammation with change in the primary and secondary outcomes using Spearman's correlations.
Results: In total, n=66 subjects completed the protocol with the primary outcome; 80% female, mean RA duration 7.7 years, 69% seropositive. The mean estimated baseline 10-year estimated ASCVD risk was 5.6%, yet 47% had evidence of CMD. We observed a significant reduction in all inflammatory markers between baseline and follow-up, as well as improvements in RA disease activity (Table 1); however, we did not observe a significant change in CFR and hs-cTnT . No correlations were observed between change in inflammatory marker with change in CFR. A reduction in hsCRP and IL-1beta was correlated with a reduction in hs-cTnT (Table 2). A non-significant trend for correlation of reduction of IL-1beta with higher CFR was observed among subjects with evidence of CMD at baseline (Table 3).
Conclusion: In one of the largest cohorts of RA subjects with detailed data on inflammatory markers, subclinical markers of myocardial injury, and CFR, reducing inflammation was correlated with improvements in measures of CV risk in a subset of patients with evidence of subclinical myocardial injury and potentially CMD via the IL-1beta pathway. In this population of overall low/borderline ASCVD risk, blood and imaging markers may inform which RA patients may benefit from CV risk reduction by targeting inflammation.
.jpg) Table 1. Change in inflammatory markers, disease activity, and measure of CV risk at baseline and follow-up, all values shown in median (interquartile range).
Table 1. Change in inflammatory markers, disease activity, and measure of CV risk at baseline and follow-up, all values shown in median (interquartile range). Table 2. Correlation between change in inflammatory markers with change in hs-cTnT.
Table 2. Correlation between change in inflammatory markers with change in hs-cTnT.  Table 3. Correlation between change in inflammatory markers with change in CFR, among subjects with CMD, n=31 (CFR < 2.5).
Table 3. Correlation between change in inflammatory markers with change in CFR, among subjects with CMD, n=31 (CFR < 2.5).Disclosures: b. weber, Horizon Therapeutics; D. Weisenfeld, None; T. Seyok, None; E. Massarotti, None; S. Huang, None; D. Todd, None; J. Coblyn, None; m. weinblatt, AbbVie/Abbott, Acclaris, Bristol Myers Squibb, Corevitas, egrx, Genosco, Horizon Therapeutics, GlaxoSmithKlein(GSK), Gilead, johnson and johnson, Eli Lilly, Pfizer, Roche, Scipher, Sanofi, set point, Tremeau, Bristol Myers Squibb, sanofi, Amgen, Eli Lilly, Canfite, Inmedix, Scipher; T. Cai, None; K. Dahal, None; l. BArrett, None; D. Solomon, AbbVie/Abbott, Amgen, moderna, CorEvitas; J. Plutzky, Amgen, Boehringer-Ingelheim, Novo Nordisk; M. Bolster, Johnson and Johnson, Merck Manual, Cumberland, Corbus Inc., PracticeUpdate, Rheumatology Research Foundation; m. Dicarli, Gilead Sciences; K. Liao, None.

