Back
Poster Session B
Juvenile idiopathic arthritis (JIA) and pediatric joint disorders
Session: (0850–0880) Pediatric Rheumatology – Clinical Poster I: JIA
0864: Baseline Clinical and Laboratory Features of the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Systemic Juvenile Idiopathic Arthritis-Associated Lung Disease (SJIA-LD) Cohort
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- EE
Esraa Eloseily, MD
Cincinnati Children's Hospital Medical Center
Cincinnati, OH, United States
Abstract Poster Presenter(s)
Esraa Eloseily1, Min-Lee Chang2, MaryEllen Riordan3, Alan Russell4, Marc Natter2, Yukiko Kimura5 and Grant Schulert6, 1Division of Pediatric Rheumatology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, 2Boston Children's Hospital, Boston, MA, 3Hackensack Meridian Health, Hackensack, NJ, 4Duke Clinical Research Institute, Duke, NC, 5Hackensack Meridian Health, New York, NY, 6Cincinnati Children's Hospital Medical Center, Cincinnati, OH
Background/Purpose: Systemic juvenile idiopathic arthritis (SJIA) associated lung disease (SJIA-LD) is an emerging and life threatening clinical problem, and currently affects as many as 1 in 20 SJIA patients. Despite recent advances, there remain key unanswered questions regarding disease prevalence, influence of biologic treatments, pathogenesis, and outcomes.
Methods: Existing or newly enrolled CARRA Registry patients with SJIA and suspected, probable, or definite SJIA-LD were included in the cohort. In addition to standard registry data, lung disease specific clinical data was obtained at baseline and at 6 month follow-up using a standardized case report form through REDCap Cloud. This study was approved by the DCRI Reliant IRB and/or IRB of all Registry sites.
Results: As of May 1, 2022, 24 patients were enrolled in the SJIA-LD cohort from 12 CARRA Registry sites. 58% were female and median age at enrollment was 4.5 (IQR 6.5). 55% had definite (biopsy-proven) SJIA-LD, 36% probable SJIA-LD, and 9% suspected SJIA-LD. Median SJIA duration at time of LD diagnosis was 1.8 years. Of those with available supplemental LD data, 70% had at least one definite prior MAS episode, 10% had probable prior MAS and 70% had more than one episode. MAS meeting 2016 SJIA-MAS criteria occurred in 50% and 30% had subclinical MAS. MAS occurred prior to LD diagnosis in 50% and coincided with it in 20%. Most common clinical features were tachypnea (60%), clubbing (50%), cough (40%) and finger and toe erythema (40%). 20% had hypoxemia requiring supplemental oxygen. Most common imaging findings were bronchial wall or peribronchovascular thickening (63%), ground-glass opacities (60%), septal thickening (56%), and hilar adenopathy (50%). On patients with available pulmonary function test data, all had abnormal DLCO, 83% had abnormal spirometry, and all had normal spot pulse oximetry. 67% of those with available BAL data had signs of pulmonary alveolar proteinosis (PAP). 30% underwent lung biopsy and all had signs of PAP and interstitial inflammation and one (33%) had collagenous fibrosis. Most recent selected lab results are shown in the table. Median IL-18 was 24,339 pg/mL (IQR: 33,173). 68% remain on oral steroids. Most commonly used biologics are Anakinra (77%), Tofacitinib (54%), Canakinumab (50%) and Tocilizumab (50%). Median Physician Global Assessment of Lung Disease (PGALD) was 3.5 (range 0- 8/10), parent/subject-reported assessment of disease activity was 2.9 (range 0-5), and health related quality of life at most recent visit was excellent in 7.7%, very good in 46, good in 7.7%, and fair in 38.4%.
Conclusion: The CARRA SJIA-LD cohort is actively enrolling patients and collecting biosamples. Median SJIA duration at time of LD diagnosis was 1.8 years. Most common features are tachypnea, clubbing, cough and finger and toe erythema. PAP seen on BAL of 67% and pathologic evidence of PAP was in all who underwent biopsy. Ongoing goals include fully characterizing clinical features of the SJIA-LD, disease progression, and defining immune biomarkers and cellular populations associated with disease trajectory. This cohort will serve as an ongoing prospective cohort study for future clinical and translational research in this emerging disease.
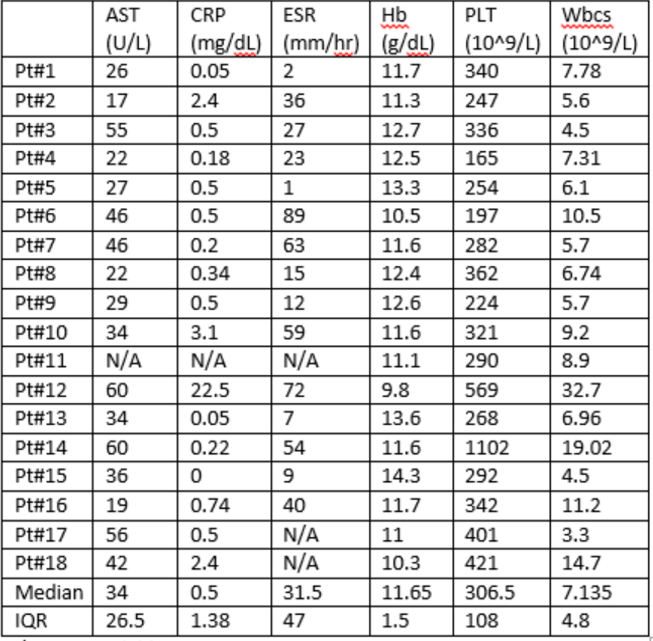 Most recent selected laboratory results. N/A: not available
Most recent selected laboratory results. N/A: not available
Disclosures: E. Eloseily, None; M. Chang, None; M. Riordan, None; A. Russell, None; M. Natter, None; Y. Kimura, Genentech; G. Schulert, Novartis, SOBI.
Background/Purpose: Systemic juvenile idiopathic arthritis (SJIA) associated lung disease (SJIA-LD) is an emerging and life threatening clinical problem, and currently affects as many as 1 in 20 SJIA patients. Despite recent advances, there remain key unanswered questions regarding disease prevalence, influence of biologic treatments, pathogenesis, and outcomes.
Methods: Existing or newly enrolled CARRA Registry patients with SJIA and suspected, probable, or definite SJIA-LD were included in the cohort. In addition to standard registry data, lung disease specific clinical data was obtained at baseline and at 6 month follow-up using a standardized case report form through REDCap Cloud. This study was approved by the DCRI Reliant IRB and/or IRB of all Registry sites.
Results: As of May 1, 2022, 24 patients were enrolled in the SJIA-LD cohort from 12 CARRA Registry sites. 58% were female and median age at enrollment was 4.5 (IQR 6.5). 55% had definite (biopsy-proven) SJIA-LD, 36% probable SJIA-LD, and 9% suspected SJIA-LD. Median SJIA duration at time of LD diagnosis was 1.8 years. Of those with available supplemental LD data, 70% had at least one definite prior MAS episode, 10% had probable prior MAS and 70% had more than one episode. MAS meeting 2016 SJIA-MAS criteria occurred in 50% and 30% had subclinical MAS. MAS occurred prior to LD diagnosis in 50% and coincided with it in 20%. Most common clinical features were tachypnea (60%), clubbing (50%), cough (40%) and finger and toe erythema (40%). 20% had hypoxemia requiring supplemental oxygen. Most common imaging findings were bronchial wall or peribronchovascular thickening (63%), ground-glass opacities (60%), septal thickening (56%), and hilar adenopathy (50%). On patients with available pulmonary function test data, all had abnormal DLCO, 83% had abnormal spirometry, and all had normal spot pulse oximetry. 67% of those with available BAL data had signs of pulmonary alveolar proteinosis (PAP). 30% underwent lung biopsy and all had signs of PAP and interstitial inflammation and one (33%) had collagenous fibrosis. Most recent selected lab results are shown in the table. Median IL-18 was 24,339 pg/mL (IQR: 33,173). 68% remain on oral steroids. Most commonly used biologics are Anakinra (77%), Tofacitinib (54%), Canakinumab (50%) and Tocilizumab (50%). Median Physician Global Assessment of Lung Disease (PGALD) was 3.5 (range 0- 8/10), parent/subject-reported assessment of disease activity was 2.9 (range 0-5), and health related quality of life at most recent visit was excellent in 7.7%, very good in 46, good in 7.7%, and fair in 38.4%.
Conclusion: The CARRA SJIA-LD cohort is actively enrolling patients and collecting biosamples. Median SJIA duration at time of LD diagnosis was 1.8 years. Most common features are tachypnea, clubbing, cough and finger and toe erythema. PAP seen on BAL of 67% and pathologic evidence of PAP was in all who underwent biopsy. Ongoing goals include fully characterizing clinical features of the SJIA-LD, disease progression, and defining immune biomarkers and cellular populations associated with disease trajectory. This cohort will serve as an ongoing prospective cohort study for future clinical and translational research in this emerging disease.
 Most recent selected laboratory results. N/A: not available
Most recent selected laboratory results. N/A: not availableDisclosures: E. Eloseily, None; M. Chang, None; M. Riordan, None; A. Russell, None; M. Natter, None; Y. Kimura, Genentech; G. Schulert, Novartis, SOBI.

