Back
Poster Session B
Infection-related rheumatic syndromes
Session: (0779–0806) Infection-related Rheumatic Disease Poster
0783: Breakthrough SARS-CoV-2 Infections and Predicting Severe Outcomes During Rituximab Therapy in Autoimmune Rheumatic Diseases
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- MM
Md Yuzaiful Md Yusof, PhD, MBChB, MRCP
University of Leeds
Leeds, United Kingdom
Abstract Poster Presenter(s)
Md Yuzaiful Md Yusof1, Jack Arnold2, Benazir Saleem3, Claire Vandevelde3, Shouvik Dass3, Sinisa Savic1, Edward M Vital1 and Paul Emery1, 1Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, United Kingdom, 2University of Leeds, Leeds, United Kingdom, 3Rheumatology Department, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom
Background/Purpose: There are concerns regarding the safety of rituximab (RTX) during the COVID-19 pandemic due its effect on humoral immunity. Data from registries during pre-vaccination period reported increased risk of poor outcomes in RTX-treated patients vs TNFi. However, registry data could be limited by reporting bias in determining true incidence. Our study objectives were to assess the incidence of breakthrough COVID infections and identify predictors of severe outcomes in RTX-treated autoimmune rheumatic diseases (ARDs), with a view to establishing a treatment algorithm for safe RTX administration.
Methods: An observational cohort study was undertaken in all 365 RTX-treated ARD patients in a single centre between index date 01/09/2019 (i.e. 6 months pre-pandemic) and 01/03/2022. Only positive cases either on a lateral flow test or PCR were included. COVID outcomes were categorised as Mild (i.e. not hospitalised) or Moderate/Severe (i.e. hospitalised or death). Predictors of moderate/severe outcomes were analysed using Cox-regression proportional hazard.
Results: Mean (SD) at index date was 59 (15) years, 274/365 (75%) patients were female and 309 (85%) were Caucasians. The diagnoses were RA=255 (70%), SLE=43 (12%), AAV=29 (8%), Sjogren=12 (3%), IIM=12 (3%) and others=14 (4%). Therapy included concomitant DMARDs = 246 (64%) and oral prednisolone = 97 (27%). Median (range) no. of previous RTX courses was 4 (0-19). A total of 720 RTX courses were administered. Of 361 patients with available outcome data, 19 (5.3%) were unvaccinated, 5 (1.4%) had a single dose, 44 (12.2%) were double-vaccinated, 207 (57.3%) triple-vaccinated and 86 (23.8%) quadruple-vaccinated. Of those who were vaccinated, for the first dose, 15% were vaccinated ≥ 6 weeks prior to cycle 1 RTX; 9.9% within 12 weeks post-RTX, 14.9% within 26 weeks and 40.3% were vaccinated >26 weeks post-RTX. Over 839 PY follow-up, 23/365 patients (6.3%) had moderate/severe COVID including 3 deaths. The rate of overall COVID and moderate/severe infections were 14.5/100 PY and 2.7/100 PY respectively. Vaccinated patients had lower rate of moderate/severe infection (3.2/100 PYs) vs unvaccinated (19.4/100 PYs) [Table 1]. The former rate was also comparable to any severe infection episode (SIE) rate in this cohort (5/100 PY). Factors associated with time-to-infection in multivariable analysis were number of comorbidities [HR 1.57 (95% CI 1.17-2.12)] and low IgG (< 6g/L) [5.54 (1.85-16.57)]. The hazard was reduced by 50% with each number of vaccine received [0.54 (0.37-0.78)]. Demographics including concomitant prednisolone, RTX- and vaccine-associated factors (e.g. RTX dose, time from RTX to vaccine, vaccine mode, peripheral B-cell depletion) were not predictive.
Conclusion: Our study allowed detailed analyses of patients over various phases of the pandemic. In the later pandemic, COVID infection rate was high but severe infection was comparable to the pre-pandemic SIE rate if vaccinated. Risk-Benefit ratios may still favour RTX in vaccinated patients with severe ARD who have limited other treatment options. Increased vigilance is needed in presence of comorbidities and low IgG level for all infection types.
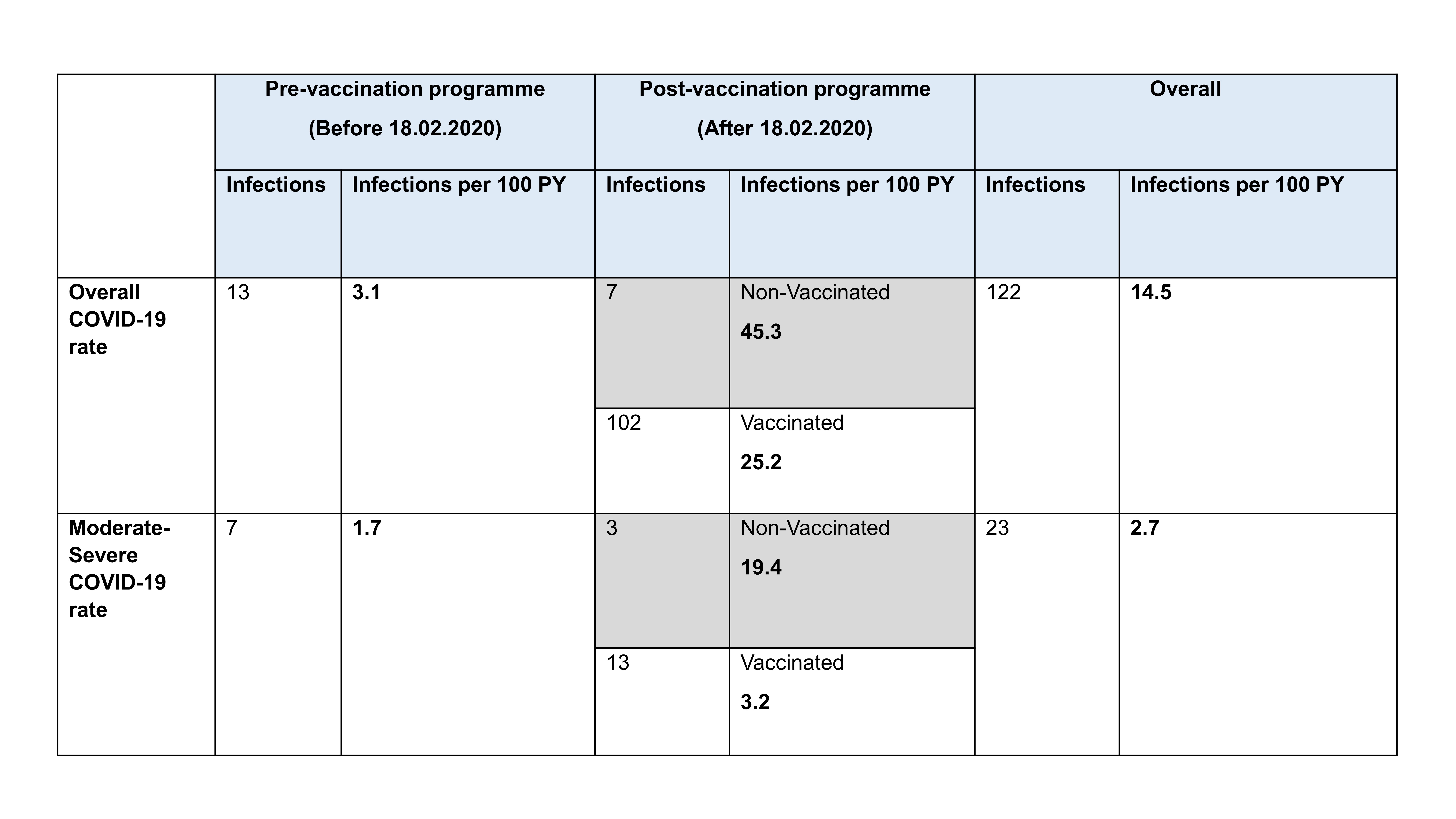 Table 1
Table 1
Disclosures: M. Md Yusof, AbbVie/Abbott, Aurinia; J. Arnold, None; B. Saleem, None; C. Vandevelde, None; S. Dass, None; S. Savic, Novartis, Swedish Orphan Biovitrum, Sire, Octapharma, CSL Behring; E. Vital, Sandoz, AstraZeneca, Eli Lilly, UCB, Novartis, Ostuka, Modus Therapeutics, Aurinia; P. Emery, AbbVie, Amgen, Bristol Myers Squibb, Celltrion, Eli Lilly, Gilead, Novartis, Pfizer, Roche, Samsung.
Background/Purpose: There are concerns regarding the safety of rituximab (RTX) during the COVID-19 pandemic due its effect on humoral immunity. Data from registries during pre-vaccination period reported increased risk of poor outcomes in RTX-treated patients vs TNFi. However, registry data could be limited by reporting bias in determining true incidence. Our study objectives were to assess the incidence of breakthrough COVID infections and identify predictors of severe outcomes in RTX-treated autoimmune rheumatic diseases (ARDs), with a view to establishing a treatment algorithm for safe RTX administration.
Methods: An observational cohort study was undertaken in all 365 RTX-treated ARD patients in a single centre between index date 01/09/2019 (i.e. 6 months pre-pandemic) and 01/03/2022. Only positive cases either on a lateral flow test or PCR were included. COVID outcomes were categorised as Mild (i.e. not hospitalised) or Moderate/Severe (i.e. hospitalised or death). Predictors of moderate/severe outcomes were analysed using Cox-regression proportional hazard.
Results: Mean (SD) at index date was 59 (15) years, 274/365 (75%) patients were female and 309 (85%) were Caucasians. The diagnoses were RA=255 (70%), SLE=43 (12%), AAV=29 (8%), Sjogren=12 (3%), IIM=12 (3%) and others=14 (4%). Therapy included concomitant DMARDs = 246 (64%) and oral prednisolone = 97 (27%). Median (range) no. of previous RTX courses was 4 (0-19). A total of 720 RTX courses were administered. Of 361 patients with available outcome data, 19 (5.3%) were unvaccinated, 5 (1.4%) had a single dose, 44 (12.2%) were double-vaccinated, 207 (57.3%) triple-vaccinated and 86 (23.8%) quadruple-vaccinated. Of those who were vaccinated, for the first dose, 15% were vaccinated ≥ 6 weeks prior to cycle 1 RTX; 9.9% within 12 weeks post-RTX, 14.9% within 26 weeks and 40.3% were vaccinated >26 weeks post-RTX. Over 839 PY follow-up, 23/365 patients (6.3%) had moderate/severe COVID including 3 deaths. The rate of overall COVID and moderate/severe infections were 14.5/100 PY and 2.7/100 PY respectively. Vaccinated patients had lower rate of moderate/severe infection (3.2/100 PYs) vs unvaccinated (19.4/100 PYs) [Table 1]. The former rate was also comparable to any severe infection episode (SIE) rate in this cohort (5/100 PY). Factors associated with time-to-infection in multivariable analysis were number of comorbidities [HR 1.57 (95% CI 1.17-2.12)] and low IgG (< 6g/L) [5.54 (1.85-16.57)]. The hazard was reduced by 50% with each number of vaccine received [0.54 (0.37-0.78)]. Demographics including concomitant prednisolone, RTX- and vaccine-associated factors (e.g. RTX dose, time from RTX to vaccine, vaccine mode, peripheral B-cell depletion) were not predictive.
Conclusion: Our study allowed detailed analyses of patients over various phases of the pandemic. In the later pandemic, COVID infection rate was high but severe infection was comparable to the pre-pandemic SIE rate if vaccinated. Risk-Benefit ratios may still favour RTX in vaccinated patients with severe ARD who have limited other treatment options. Increased vigilance is needed in presence of comorbidities and low IgG level for all infection types.
 Table 1
Table 1Disclosures: M. Md Yusof, AbbVie/Abbott, Aurinia; J. Arnold, None; B. Saleem, None; C. Vandevelde, None; S. Dass, None; S. Savic, Novartis, Swedish Orphan Biovitrum, Sire, Octapharma, CSL Behring; E. Vital, Sandoz, AstraZeneca, Eli Lilly, UCB, Novartis, Ostuka, Modus Therapeutics, Aurinia; P. Emery, AbbVie, Amgen, Bristol Myers Squibb, Celltrion, Eli Lilly, Gilead, Novartis, Pfizer, Roche, Samsung.

