Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0589–0628) RA – Etiology and Pathogenesis Poster
0605: Citrullinated Peptide-Specific ACPA Are Present in the Female Genital Tract in Premenopausal Women with and Without RA
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- DM
Daniele Marcy, MD, BS
University of Colorado
Aurora, CO, United States
Abstract Poster Presenter(s)
Daniele Marcy1, Heather Rothfuss2, Ashley Visser1, Jill Norris3, V. Michael Holers4, Kevin D Deane5, William Robinson6, Brian Cherrington2 and Kristen Demoruelle1, 1University of Colorado Anschutz Medical Campus, Aurora, CO, 2University of Wyoming, Laramie, 3Colorado School of Public Health, Aurora, CO, 4University of Colorado, Denver, CO, 5University of Colorado Denver Anschutz Medical Campus, Denver, CO, 6Stanford University School of Medicine, Palo Alto, CA
Background/Purpose: Women develop RA ~3 times more often than men. The etiology of this sex difference remains largely unexplained. Generation of anti-citrullinated protein antibodies (ACPA) has been demonstrated at mucosal sites in individuals with RA and those at-risk for developing RA (At-Risk). However, most of the investigation in this area has focused on the lung, gut and gingival mucosae, and none of this work has explained sex differences in RA. Our group previously reported that anti-CCP-IgG antibodies are detectable in the female genital tract of premenopausal women with and without RA. Herein, we investigate citrullinated peptide-specific ACPA in the female genital tract of premenopausal women with and without RA
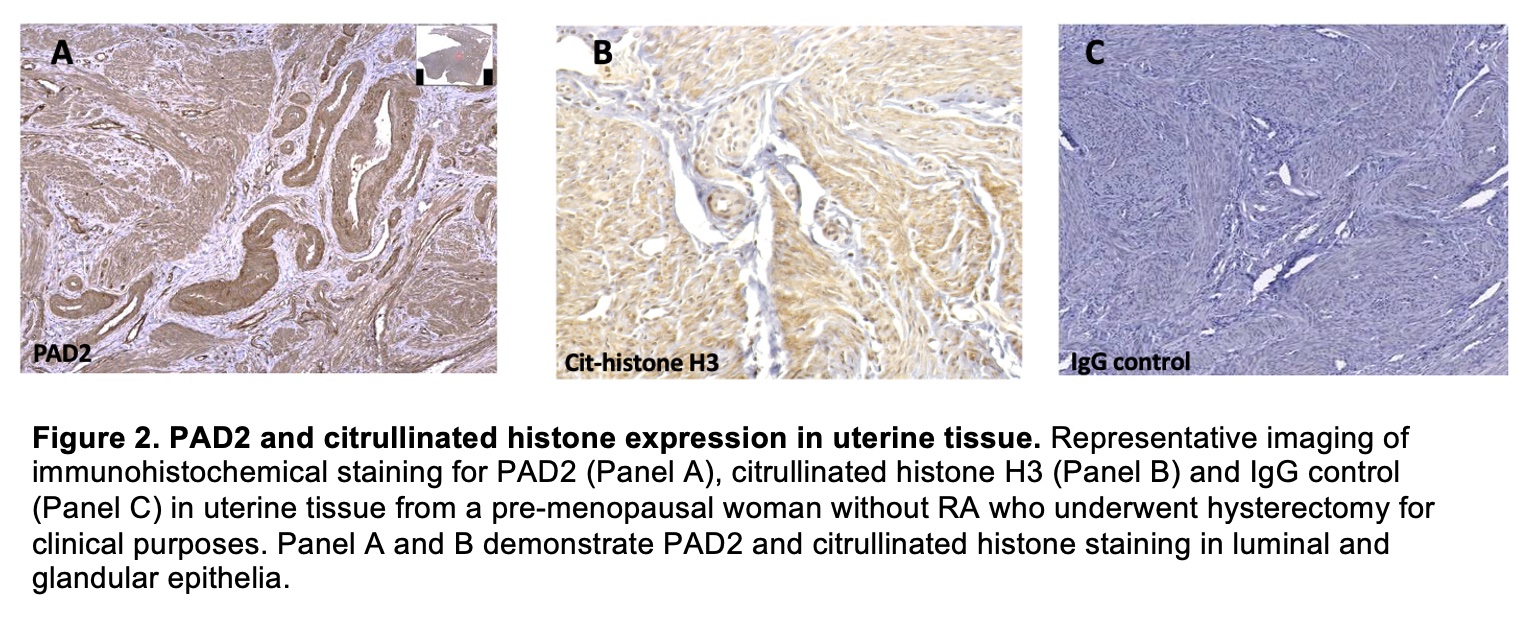
Methods: We studied premenopausal women with classified RA (n=12), At-Risk for RA based on familial risk (n=12; 2 of 12 also serum anti-CCP+) and serum anti-CCP negative healthy controls (n=32). Cervicovaginal fluid (CVF) was self-collected by participants. Paired serum and CVF were tested for IgG directed to 10 pairs of citrullinated and native peptides using a bead-based array. Citrullinated peptide-specific ACPA were defined by antibody reactivity to the citrullinated peptide greater than reactivity to the native peptide. We also performed immunohistochemical (IHC) staining for peptidylarginine deiminase (PAD)-2, an enzyme that citrullinates proteins, and histone H3, a known target of PAD2 with an established commercial antibody available. PAD2 and citrullinated histones are both associated with RA and were examined in 10 uterine tissue samples obtained from pre-menopausal women without RA following hysterectomy.
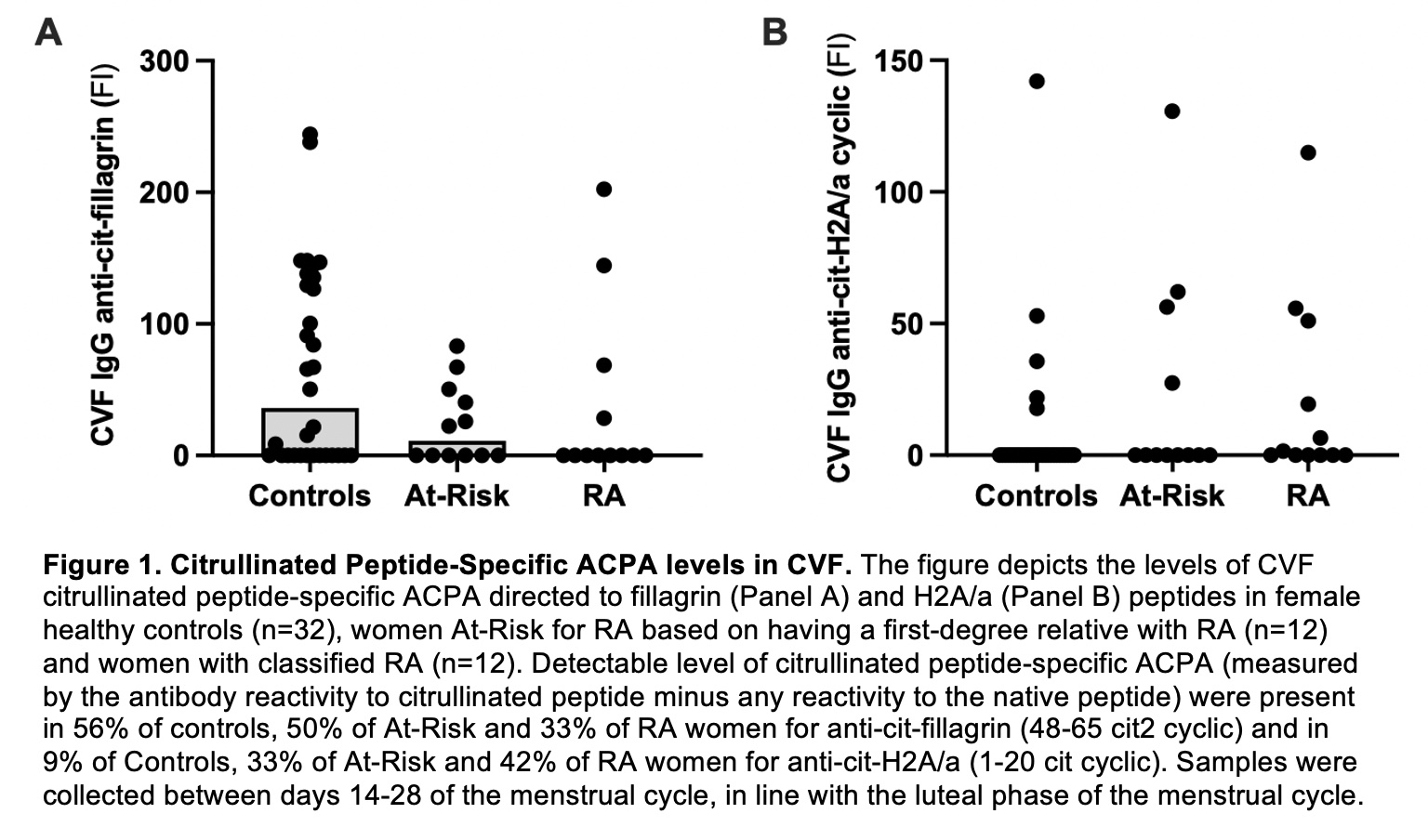
Results: Citrullinated-peptide specific CVF ACPA directed to fillagrin (48-65 cit2 cyclic) was highly prevalent across all groups (33% RA, 50% At-Risk, 56% Controls, Figure 1), whereas citrullinated peptide-specific CVF ACPA directed to histone H2A/a (1-20 cit cyclic) was more prevalent in RA (42% RA, 33% At-Risk, 9% Controls, p=0.04, Figure 1). CVF citrullinated peptide-specific ACPA was not associated with age, smoking, contraception use or pregnancy history. There was no correlation between citrullinated peptide-specific ACPA levels in CVF and paired serum in any group. IHC staining demonstrated presence of PAD2 and citrullinated histones in all uterine tissue samples (Figure 2).
Conclusion: We demonstrate for the first time that citrullinated-peptide specific ACPA are present in the female genital tract mucosa of pre-menopausal women with and without RA. While some CVF ACPA were highly prevalent in all women (i.e., anti-cit-fillagrin), some CVF ACPA were specific for women with RA and At-Risk for RA (i.e., anti-cit-H2A/a). We also found that PAD2 and citrullinated histone are highly expressed in uterine tissue of premenopausal women, supporting that the enzymes and citrullinated peptides that can serve as antigenic targets of ACPA are present locally in the female genital tract. Additional studies are needed to determine whether local ACPA generation in the female genital tract can transition to systemic ACPA in some women, which could implicate immune dysregulation of this female-specific mucosal site in the sex differences seen in RA.
Disclosures: D. Marcy, None; H. Rothfuss, None; A. Visser, None; J. Norris, None; V. Holers, Janssen; K. Deane, Werfen; W. Robinson, None; B. Cherrington, None; K. Demoruelle, Boehringer-Ingelheim, Pfizer.
Background/Purpose: Women develop RA ~3 times more often than men. The etiology of this sex difference remains largely unexplained. Generation of anti-citrullinated protein antibodies (ACPA) has been demonstrated at mucosal sites in individuals with RA and those at-risk for developing RA (At-Risk). However, most of the investigation in this area has focused on the lung, gut and gingival mucosae, and none of this work has explained sex differences in RA. Our group previously reported that anti-CCP-IgG antibodies are detectable in the female genital tract of premenopausal women with and without RA. Herein, we investigate citrullinated peptide-specific ACPA in the female genital tract of premenopausal women with and without RA
Methods: We studied premenopausal women with classified RA (n=12), At-Risk for RA based on familial risk (n=12; 2 of 12 also serum anti-CCP+) and serum anti-CCP negative healthy controls (n=32). Cervicovaginal fluid (CVF) was self-collected by participants. Paired serum and CVF were tested for IgG directed to 10 pairs of citrullinated and native peptides using a bead-based array. Citrullinated peptide-specific ACPA were defined by antibody reactivity to the citrullinated peptide greater than reactivity to the native peptide. We also performed immunohistochemical (IHC) staining for peptidylarginine deiminase (PAD)-2, an enzyme that citrullinates proteins, and histone H3, a known target of PAD2 with an established commercial antibody available. PAD2 and citrullinated histones are both associated with RA and were examined in 10 uterine tissue samples obtained from pre-menopausal women without RA following hysterectomy.
Results: Citrullinated-peptide specific CVF ACPA directed to fillagrin (48-65 cit2 cyclic) was highly prevalent across all groups (33% RA, 50% At-Risk, 56% Controls, Figure 1), whereas citrullinated peptide-specific CVF ACPA directed to histone H2A/a (1-20 cit cyclic) was more prevalent in RA (42% RA, 33% At-Risk, 9% Controls, p=0.04, Figure 1). CVF citrullinated peptide-specific ACPA was not associated with age, smoking, contraception use or pregnancy history. There was no correlation between citrullinated peptide-specific ACPA levels in CVF and paired serum in any group. IHC staining demonstrated presence of PAD2 and citrullinated histones in all uterine tissue samples (Figure 2).
Conclusion: We demonstrate for the first time that citrullinated-peptide specific ACPA are present in the female genital tract mucosa of pre-menopausal women with and without RA. While some CVF ACPA were highly prevalent in all women (i.e., anti-cit-fillagrin), some CVF ACPA were specific for women with RA and At-Risk for RA (i.e., anti-cit-H2A/a). We also found that PAD2 and citrullinated histone are highly expressed in uterine tissue of premenopausal women, supporting that the enzymes and citrullinated peptides that can serve as antigenic targets of ACPA are present locally in the female genital tract. Additional studies are needed to determine whether local ACPA generation in the female genital tract can transition to systemic ACPA in some women, which could implicate immune dysregulation of this female-specific mucosal site in the sex differences seen in RA.


Disclosures: D. Marcy, None; H. Rothfuss, None; A. Visser, None; J. Norris, None; V. Holers, Janssen; K. Deane, Werfen; W. Robinson, None; B. Cherrington, None; K. Demoruelle, Boehringer-Ingelheim, Pfizer.

