Back
Poster Session D
Osteoarthritis (OA) and related disorders
Session: (1888–1923) Osteoarthritis – Clinical Poster
1902: Structural Severity in Knee Osteoarthritis Impacts Treatment Response: A Post Hoc Pooled Analysis of Lorecivivint Clinical Trials
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- TM
Timothy McAlindon, MD, MPH
Tufts Medical Center
Arlington, MA, United States
Abstract Poster Presenter(s)
Yusuf Yazici1, Christopher Swearingen2, Heli Ghandehari3, ismail simsek4, Sarah Kennedy5, Jeyanesh Tambiah6 and Timothy McAlindon7, 1New York University School of Medicine, La Jolla, CA, 2Samumed LLC, San Diego, CA, 3Biosplice Therapeutics, Inc., San Diego, CA, 4Alpine Immunesciences, San Diego, CA, 5Biosplice Therapeutics, Inc, San Diego, CA, 6Biosplice Ther Inc., San Diego, CA, 7Tufts Medical Center, Arlington, MA
Background/Purpose: Osteoarthritis (OA) is a leading cause of disability globally, and its disease burden is expected to increase as risk factors such as aging and obesity also rise (Wallace, IJ et al. PNAS 2017). Unmet needs remain for safe and efficacious treatments for both symptoms and structural modification. Disease heterogeneity and the difficulties assessing pain and joint structure in clinical trials are challenges to developing effective treatments options. This post hoc analysis of lorecivivint (LOR), an intra-articular (IA) CLK/DYRK inhibitor that modulates Wnt and inflammatory pathways, examined the structural heterogeneity of participants enrolled in Phase 2 and 3 LOR trials and then the associated treatment responses within KL grade subgroups. The purpose of this analysis was to identify potential relationships between OA pain and knee joint structure which may aid future clinical trial design.
Methods: Data was analyzed from two Phase 2 (OA-02, NCT02536833; OA-04, NCT03122860), and two Phase 3 trials (OA-10, NCT04385303; OA-11, NCT03928184). In all trials, participants had ACR-defined (clinical and radiographic) knee OA, Kellgren-Lawrence (KL) grades 2-3. For OA-04, OA-10 and OA-11, additional criteria included Pain Numeric Rating Scale (NRS) [0-10] ≥4 and ≤8 in the target knee and < 4 in the contralateral knee. Baseline JSW for each study was compared using a cumulative frequency distribution plots by KL grade; percentages of subjects with JSW < 3mm for each study was also summarized to provide a surrogate for loss of ~50% healthy JSW (Deep et al. JBJS 2003). For the trials which captured Pain NRS, treatment responses were assessed according to KL grade and by trial. For all treatment-related outcomes, change from baseline was estimated using baseline-adjusted ANCOVA at each timepoint.
Results: In the Phase 2 trials, 16% (OA-02) and 21% (OA-04) and in the Phase 3 trials, 30% (OA-10) and 49% (OA-11) of KL 2 participants had baseline mJSW < 3 mm. For KL 3 participants, OA-02 had 55% and OA-04 53%, while OA-10 had 81% and OA-11 88% mJSW < 3mm (figure 1). Beneficial treatment effect of LOR vs. PBO was seen in KL 2 for OA-04 and OA-10, but not OA-11, with similar magnitude of response seen for 0.07 mg and 0.23 mg doses (figure 2). Beneficial treatment effect of LOR was seen only in OA-04 for KL 3, with similar magnitude of change for 0.07 mg and 0.23 mg LOR through week 12, and greater improvement observed for the 0.23 mg dose for weeks 12-24 (figure 3).
Conclusion: In this post hoc analysis, there was substantial heterogeneity in baseline mJSWs across LOR clinical trials within the KL 2-3 grade inclusion criteria. Participants with less structurally advanced knee OA showed greater pain treatment responses to 0.07 mg LOR compared to those with more advanced disease. These data support the hypothesis that the amount of OA structural damage is associated with the pain of knee OA and that earlier intervention may improve outcomes.
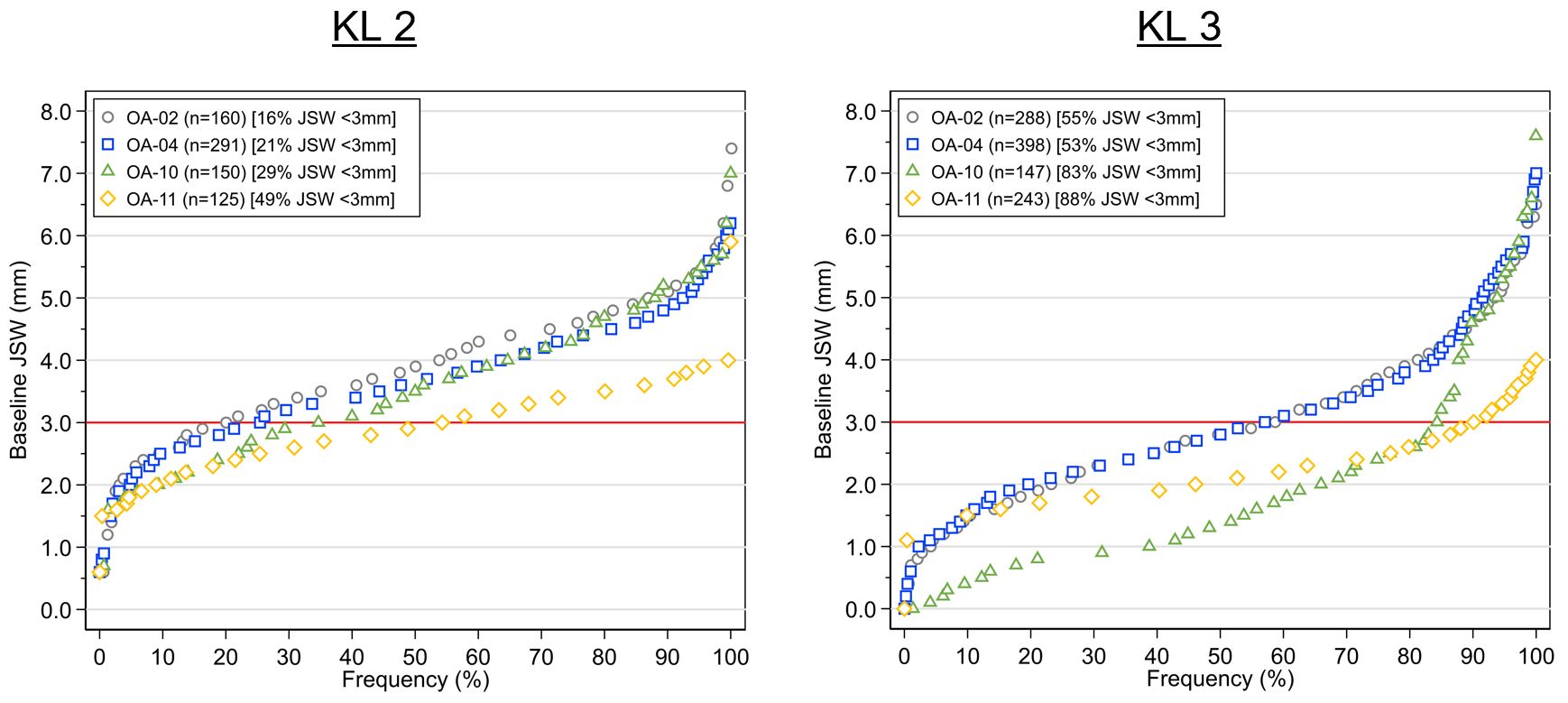 Figure 1. Cumulative frequency of baseline mJSW by KL grade across LOR trials
Figure 1. Cumulative frequency of baseline mJSW by KL grade across LOR trials
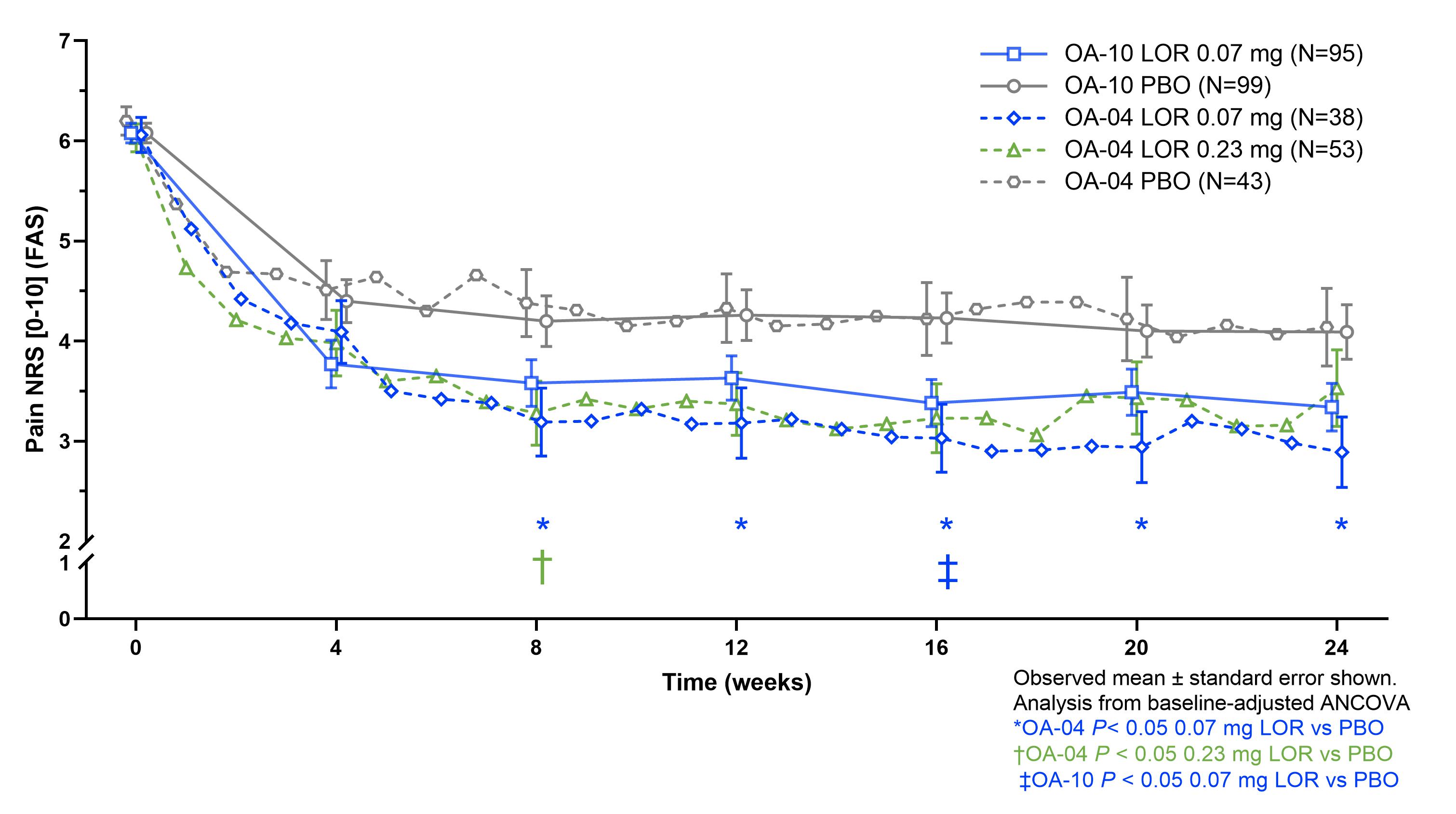 Figure 2. Treatment effect of LOR vs PBO by KL grade 2 across LOR trials
Figure 2. Treatment effect of LOR vs PBO by KL grade 2 across LOR trials
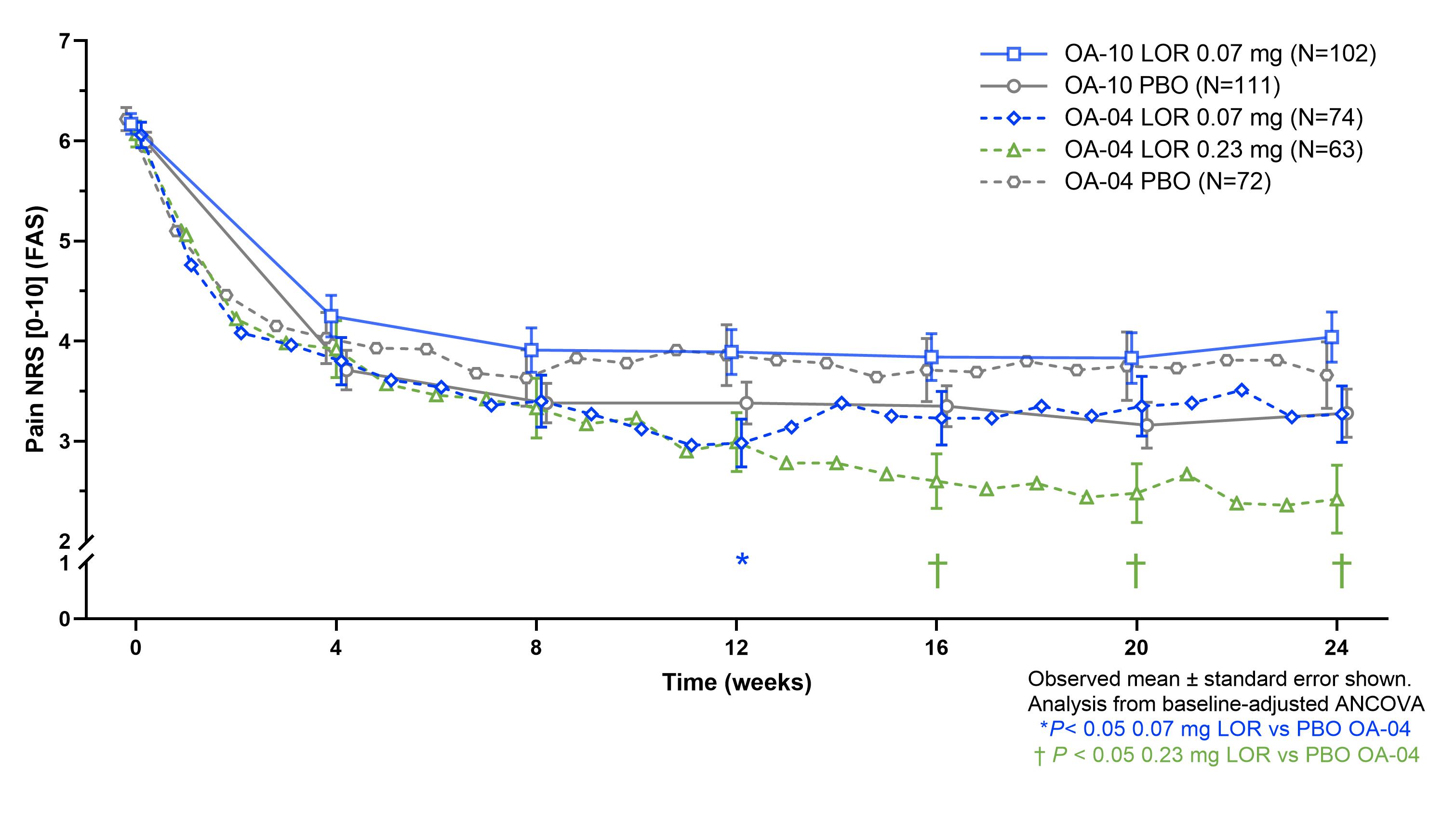 Figure 3. Treatment effect of LOR vs PBO by KL grade 3 across LOR trials
Figure 3. Treatment effect of LOR vs PBO by KL grade 3 across LOR trials
Disclosures: Y. Yazici, Amgen, Biosplice; C. Swearingen, Biosplice Therapeutics, Inc; H. Ghandehari, Biosplice Therapeutics, Inc.; i. simsek, Biosplice Inc.; S. Kennedy, Biosplice Therapeutics, Inc; J. Tambiah, Biosplice Therapeutics Inc; T. McAlindon, Biosplice Therapeutics, Inc, Remedium-Bio, Organogenesis, Pfizer, Kolon Tissue Gene, Seikugaku.
Background/Purpose: Osteoarthritis (OA) is a leading cause of disability globally, and its disease burden is expected to increase as risk factors such as aging and obesity also rise (Wallace, IJ et al. PNAS 2017). Unmet needs remain for safe and efficacious treatments for both symptoms and structural modification. Disease heterogeneity and the difficulties assessing pain and joint structure in clinical trials are challenges to developing effective treatments options. This post hoc analysis of lorecivivint (LOR), an intra-articular (IA) CLK/DYRK inhibitor that modulates Wnt and inflammatory pathways, examined the structural heterogeneity of participants enrolled in Phase 2 and 3 LOR trials and then the associated treatment responses within KL grade subgroups. The purpose of this analysis was to identify potential relationships between OA pain and knee joint structure which may aid future clinical trial design.
Methods: Data was analyzed from two Phase 2 (OA-02, NCT02536833; OA-04, NCT03122860), and two Phase 3 trials (OA-10, NCT04385303; OA-11, NCT03928184). In all trials, participants had ACR-defined (clinical and radiographic) knee OA, Kellgren-Lawrence (KL) grades 2-3. For OA-04, OA-10 and OA-11, additional criteria included Pain Numeric Rating Scale (NRS) [0-10] ≥4 and ≤8 in the target knee and < 4 in the contralateral knee. Baseline JSW for each study was compared using a cumulative frequency distribution plots by KL grade; percentages of subjects with JSW < 3mm for each study was also summarized to provide a surrogate for loss of ~50% healthy JSW (Deep et al. JBJS 2003). For the trials which captured Pain NRS, treatment responses were assessed according to KL grade and by trial. For all treatment-related outcomes, change from baseline was estimated using baseline-adjusted ANCOVA at each timepoint.
Results: In the Phase 2 trials, 16% (OA-02) and 21% (OA-04) and in the Phase 3 trials, 30% (OA-10) and 49% (OA-11) of KL 2 participants had baseline mJSW < 3 mm. For KL 3 participants, OA-02 had 55% and OA-04 53%, while OA-10 had 81% and OA-11 88% mJSW < 3mm (figure 1). Beneficial treatment effect of LOR vs. PBO was seen in KL 2 for OA-04 and OA-10, but not OA-11, with similar magnitude of response seen for 0.07 mg and 0.23 mg doses (figure 2). Beneficial treatment effect of LOR was seen only in OA-04 for KL 3, with similar magnitude of change for 0.07 mg and 0.23 mg LOR through week 12, and greater improvement observed for the 0.23 mg dose for weeks 12-24 (figure 3).
Conclusion: In this post hoc analysis, there was substantial heterogeneity in baseline mJSWs across LOR clinical trials within the KL 2-3 grade inclusion criteria. Participants with less structurally advanced knee OA showed greater pain treatment responses to 0.07 mg LOR compared to those with more advanced disease. These data support the hypothesis that the amount of OA structural damage is associated with the pain of knee OA and that earlier intervention may improve outcomes.
 Figure 1. Cumulative frequency of baseline mJSW by KL grade across LOR trials
Figure 1. Cumulative frequency of baseline mJSW by KL grade across LOR trials Figure 2. Treatment effect of LOR vs PBO by KL grade 2 across LOR trials
Figure 2. Treatment effect of LOR vs PBO by KL grade 2 across LOR trials Figure 3. Treatment effect of LOR vs PBO by KL grade 3 across LOR trials
Figure 3. Treatment effect of LOR vs PBO by KL grade 3 across LOR trialsDisclosures: Y. Yazici, Amgen, Biosplice; C. Swearingen, Biosplice Therapeutics, Inc; H. Ghandehari, Biosplice Therapeutics, Inc.; i. simsek, Biosplice Inc.; S. Kennedy, Biosplice Therapeutics, Inc; J. Tambiah, Biosplice Therapeutics Inc; T. McAlindon, Biosplice Therapeutics, Inc, Remedium-Bio, Organogenesis, Pfizer, Kolon Tissue Gene, Seikugaku.

