Back
Poster Session D
Session: (2052–2107) SLE – Diagnosis, Manifestations, and Outcomes Poster III: Outcomes
2079: Survey to Prioritize and Generate Domains in Preparation to Update the OMERACT Core Domain Set for Systemic Lupus Erythematosus
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- WN
Wils Nielsen, MSc
University of Toronto
Markham, ON, Canada
Abstract Poster Presenter(s)
Wils Nielsen1, Vibeke Strand2, Lee S Simon3, Julian Thumboo4, Marta Mosca5, Martin Aringer6, Sindu Johnson7, Aaron Drucker7, Eric Morand8, Ian N. Bruce9 and Zahi Touma1, 1Schroeder Arthritis Institute, Krembil Research Institute, University Health Network and University of Toronto, Toronto, ON, Canada, 2Stanford University School of Medicine, Stanford, CA, 3SDG LLC, West Newton, MA, 4Singapore General Hospital, Bukit Merah, Singapore, 5Rheumatology Unit, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy, 6University Medical Center and Faculty of Medicine TU Dresden, Dresden, Germany, 7University of Toronto, Toronto, ON, Canada, 8Monash University, Victoria; Department of Rheumatology, Monash Health, Melbourne, Australia, 9Centre for Epidemiology Versus Arthritis, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, United Kingdom
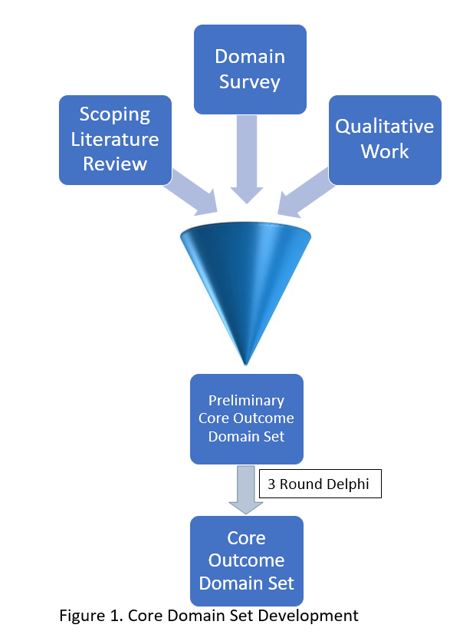
Background/Purpose: The development of a Core Outcome domain Set (COS) can aid research communication and standardizing measurement tools of SLE. A COS captures SLE facets relevant to different stakeholders (clinicians, patients, researchers, etc.) for randomized controlled trials and longitudinal observational studies. Once a COS is developed, measurement instruments are selected for each domain. The previous Outcome Measures in Rheumatology (OMERACT) SLE COS was developed in 1998. Since then, several novel, pertinent domains have been identified, creating a need to update the SLE COS. We recently established the OMERACT SLE Working Group of 152 members (including patients, physicians, researchers and other stakeholders) representing 5 continents and 25 countries. The first step in COS development is to generate an extensive preliminary list of potential domains through a) a scoping literature review, b) qualitative studies with stakeholders, and c) a domain survey. This will be followed by a Delphi process to develop the final COS from the preliminary COS [Figure 1]. In this abstract we report the results of the domain survey completed by the SLE stakeholders to prioritize a list of COS domains for consideration.
Methods: We retrieved domains endorsed for the 1998 SLE COS and other domains considered in the research agenda, adapted them to modern versions, and included additional domains deemed important by the eight OMERACT SLE Working Group Co-Chairs. The survey included a total of 18 domains and was distributed electronically to 143 members of the OMERACT SLE Working Group and an additional 33 SLE expert clinicians/researchers. Responses were anonymous. The survey had 2 major aims: 1. To agree on a list of domains for inclusion in the COS with response options (Yes, No, I don't know) and 2. to prioritize the importance of selected domains (measured on a 1-9 Likert Scale). We utilized a cut-off of ≥70% both for "Yes" for inclusion and for importance, represented by a score of 7-9 on the Likert Scale.
Results: 145 of 176 (82.4%) participants completed the survey. Total responses are reported in Table 1. The domain with the highest proportion of respondents favoring inclusion was Disease Activity (98%) and the lowest was Sleep (45%). Disease Activity, Damage, Health Related Quality of Life, Functional Ability, Use of Glucocorticoids including Demonstrated Tapering, Tolerability/Adverse Events/Death, Comorbidities, and Fatigue met criteria for further consideration, while Pain, Cognitive Function, Depressive Symptoms, Pregnancy, Work Status, Psychosocial Factors, Economic Cost, Anxiety, Frailty, and Sleep did not reach this cut-off. Domains that scored lower for inclusion had more "I don't know" responses suggesting further instruction/discussion of the domains would be beneficial.
Conclusion: In this survey of stakeholders, 8 of 18 domains met SLE COS consideration criteria. Of the remaining 10, 4 met the 70% cut-off for inclusion, but not for importance. A survey of 100 SLE patients is in progress; their responses will be combined with the results of this survey and our scoping review and qualitative work (in progress) to inform the first phase of the update of the OMERACT SLE COS.

Disclosures: W. Nielsen, None; V. Strand, AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb(BMS), Boehringer-Ingelheim, Chemocentryx, Celltrion, Genentech/Roche, Gilead, GlaxoSmithKlein(GSK), Inmedix, Janssen, Kiniksa, Merck/MSD, Novartis, Pfizer, Regeneron Pharmaceuticals, Rheos, R-Pharma, Samsung, Sandoz, Sanofi, Scipher, Setpoint, Spherix, Aria, Bioventus, Blackrock, Equilium, Glenmark, Horizon, Kypha, Lilly, MiMedx, Sorrento, Tonix, Priovant; L. Simon, None; J. Thumboo, None; M. Mosca, None; M. Aringer, AbbVie/Abbott, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Eli Lilly, GlaxoSmithKlein(GSK), Merck/MSD, Novartis, Pfizer, Roche, Galapagos, Otsuka; S. Johnson, None; A. Drucker, None; E. Morand, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Eli Lilly, Genentech, GlaxoSmithKlein(GSK), Janssen, Novartis, Servier, UCB, EMD Serono, Billerica, MA, USA; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); Z. Touma, None.
Background/Purpose: The development of a Core Outcome domain Set (COS) can aid research communication and standardizing measurement tools of SLE. A COS captures SLE facets relevant to different stakeholders (clinicians, patients, researchers, etc.) for randomized controlled trials and longitudinal observational studies. Once a COS is developed, measurement instruments are selected for each domain. The previous Outcome Measures in Rheumatology (OMERACT) SLE COS was developed in 1998. Since then, several novel, pertinent domains have been identified, creating a need to update the SLE COS. We recently established the OMERACT SLE Working Group of 152 members (including patients, physicians, researchers and other stakeholders) representing 5 continents and 25 countries. The first step in COS development is to generate an extensive preliminary list of potential domains through a) a scoping literature review, b) qualitative studies with stakeholders, and c) a domain survey. This will be followed by a Delphi process to develop the final COS from the preliminary COS [Figure 1]. In this abstract we report the results of the domain survey completed by the SLE stakeholders to prioritize a list of COS domains for consideration.
Methods: We retrieved domains endorsed for the 1998 SLE COS and other domains considered in the research agenda, adapted them to modern versions, and included additional domains deemed important by the eight OMERACT SLE Working Group Co-Chairs. The survey included a total of 18 domains and was distributed electronically to 143 members of the OMERACT SLE Working Group and an additional 33 SLE expert clinicians/researchers. Responses were anonymous. The survey had 2 major aims: 1. To agree on a list of domains for inclusion in the COS with response options (Yes, No, I don't know) and 2. to prioritize the importance of selected domains (measured on a 1-9 Likert Scale). We utilized a cut-off of ≥70% both for "Yes" for inclusion and for importance, represented by a score of 7-9 on the Likert Scale.
Results: 145 of 176 (82.4%) participants completed the survey. Total responses are reported in Table 1. The domain with the highest proportion of respondents favoring inclusion was Disease Activity (98%) and the lowest was Sleep (45%). Disease Activity, Damage, Health Related Quality of Life, Functional Ability, Use of Glucocorticoids including Demonstrated Tapering, Tolerability/Adverse Events/Death, Comorbidities, and Fatigue met criteria for further consideration, while Pain, Cognitive Function, Depressive Symptoms, Pregnancy, Work Status, Psychosocial Factors, Economic Cost, Anxiety, Frailty, and Sleep did not reach this cut-off. Domains that scored lower for inclusion had more "I don't know" responses suggesting further instruction/discussion of the domains would be beneficial.
Conclusion: In this survey of stakeholders, 8 of 18 domains met SLE COS consideration criteria. Of the remaining 10, 4 met the 70% cut-off for inclusion, but not for importance. A survey of 100 SLE patients is in progress; their responses will be combined with the results of this survey and our scoping review and qualitative work (in progress) to inform the first phase of the update of the OMERACT SLE COS.


Disclosures: W. Nielsen, None; V. Strand, AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb(BMS), Boehringer-Ingelheim, Chemocentryx, Celltrion, Genentech/Roche, Gilead, GlaxoSmithKlein(GSK), Inmedix, Janssen, Kiniksa, Merck/MSD, Novartis, Pfizer, Regeneron Pharmaceuticals, Rheos, R-Pharma, Samsung, Sandoz, Sanofi, Scipher, Setpoint, Spherix, Aria, Bioventus, Blackrock, Equilium, Glenmark, Horizon, Kypha, Lilly, MiMedx, Sorrento, Tonix, Priovant; L. Simon, None; J. Thumboo, None; M. Mosca, None; M. Aringer, AbbVie/Abbott, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Eli Lilly, GlaxoSmithKlein(GSK), Merck/MSD, Novartis, Pfizer, Roche, Galapagos, Otsuka; S. Johnson, None; A. Drucker, None; E. Morand, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Eli Lilly, Genentech, GlaxoSmithKlein(GSK), Janssen, Novartis, Servier, UCB, EMD Serono, Billerica, MA, USA; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); Z. Touma, None.

