Back
Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1004–1034) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster II
1010: Comparison of Established and New, Preliminarily Proposed ASAS Cut-Offs for Inflammatory MRI Lesions in the Sacroiliac Joints of Axial Spondyloarthritis Patients and Implications for Recruitment in Clinical Studies
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- XB
Xenofon Baraliakos, MD
Rheumazentrum Ruhrgebiet Herne
Herne, Germany
Abstract Poster Presenter(s)
Xenofon Baraliakos1, Pedro Machado2, Lars Bauer3, Bengt Hoepken3, Mindy Kim4, Thomas Kumke3, Rachel Tham5 and Martin Rudwaleit6, 1Rheumazentrum Ruhrgebiet Herne, Herne, Germany, 2University College London, London, United Kingdom, 3UCB Pharma, Monheim am Rhein, Germany, 4UCB Pharma, Smyrna, GA, 5UCB Pharma, Slough, UK, Overland Park, KS, 6University of Bielefeld, Klinikum Bielefeld, Bielefeld; Germany Klinikum Bielefeld and Charité Berlin, Germany, and Gent University, Gent, Belgium
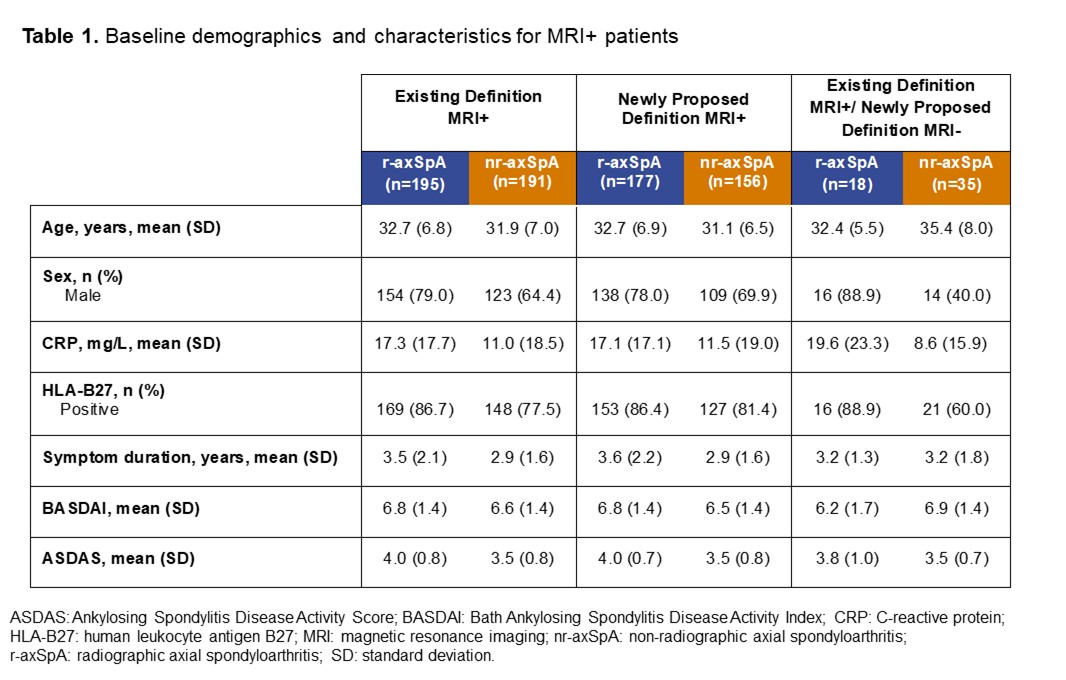
Background/Purpose: The Assessment of SpondyloArthritis international Society (ASAS) recently proposed preliminary, more stringent, data-driven definitions for active and structural MRI lesions of the sacroiliac joint (SIJ) typical of axial spondyloarthritis (axSpA).1 We assessed clinical outcomes of patients receiving certolizumab pegol (CZP) in the C-OPTIMISE trial who were MRI-positive (MRI+) or negative (MRI-) according to both the existing and newly proposed definitions, as well as those who were MRI+ by the existing definition but MRI- by the newly proposed definition (discordant group).
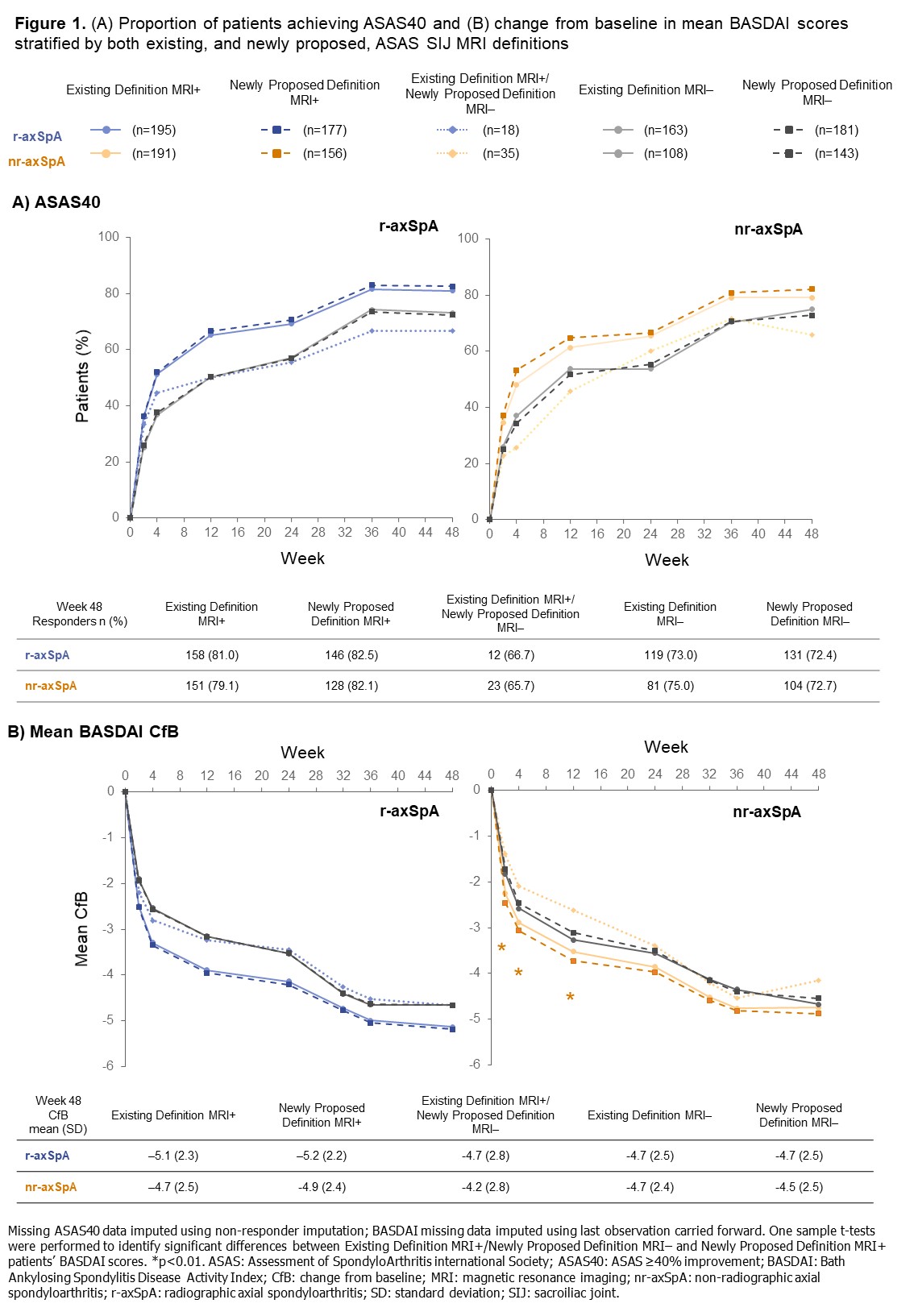
Methods: C-OPTIMISE (NCT02505542) enrolled 736 patients with active axSpA who received 400 mg CZP at Weeks 0, 2, and 4, then 200mg CZP every two weeks to Week 48.2 We report: percentage of patients achieving ASAS ≥40% improvement (ASAS40) response; mean BASDAI change from baseline (CfB) and Ankylosing Spondylitis Disease Activity Score (ASDAS) disease state to Week 48. In this post hoc analysis, outcomes were stratified by axSpA subgroup (radiographic [r-]/ non-radiographic [nr]-axSpA) and MRI definition. Missing values were imputed using non-responder imputation for ASAS40 and last observation carried forward for continuous variables.
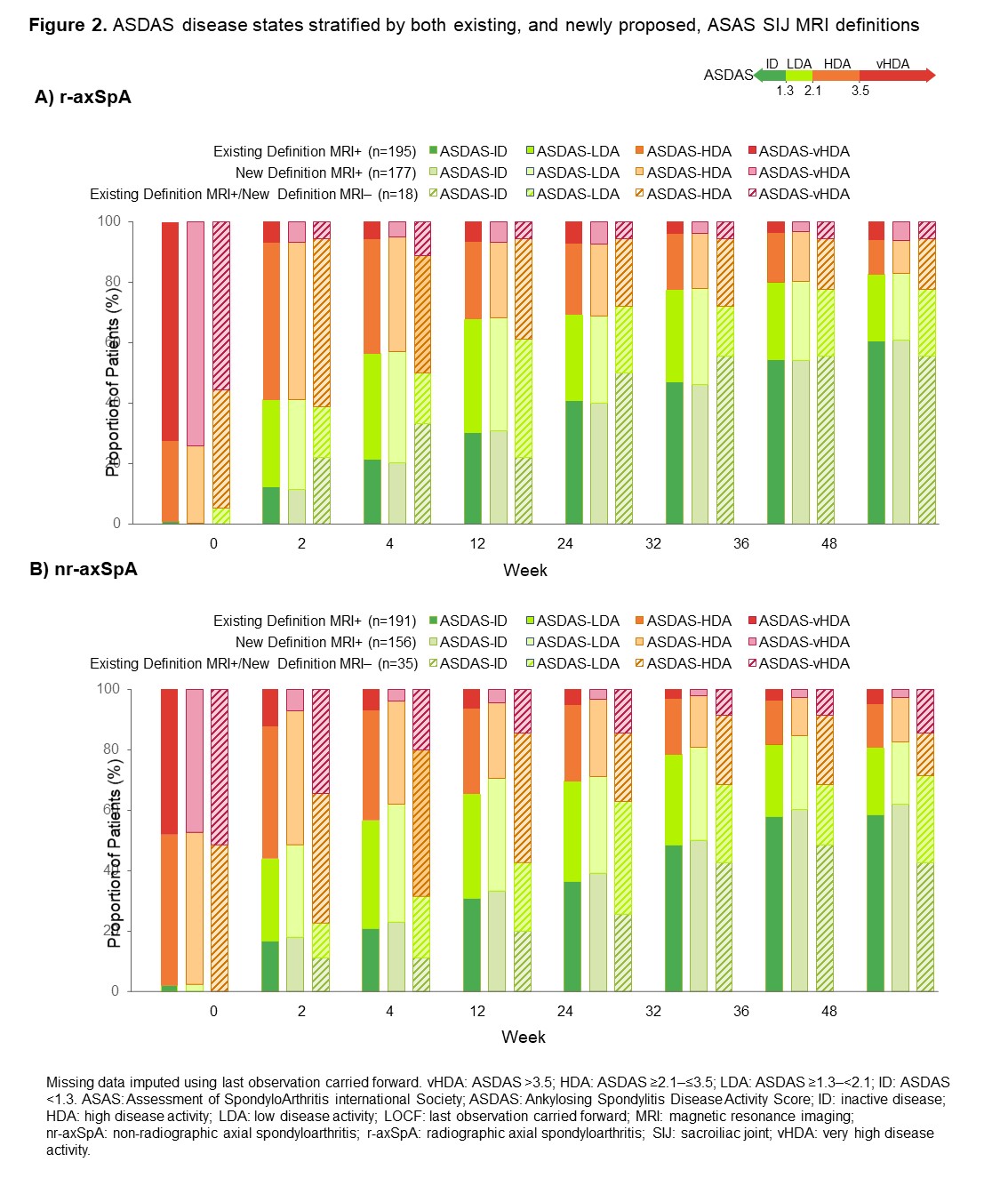
Results: Baseline MRI data were available for 657/736 (89.3%) patients, 358/657 (54.5%) of whom had r-axSpA and 299/657 (45.5%) nr-axSpA. 386/657 patients (58.8%) were classified as MRI+ according to the existing definition compared with 333/657 patients (50.7%) using the newly proposed definition; the discordant group comprised 53/657 patients (8.1%; nr-axSpA: 35/299 [11.7%]; r-axSpA: 18/358 [5.0%]; Table 1). A numerically higher proportion of newly proposed definition MRI+ patients achieved ASAS40 at Week 48 versus those fulfilling the existing MRI+ definition; patients in the discordant group responded similarly to those originally classified as MRI- (Figure 1A). Similar results were observed for mean BASDAI, with significant differences in responses between nr-axSpA patients in the newly proposed MRI+ definition and discordant groups (Figure 1B). At Week 48, a lower proportion of patients in the discordant group achieved ASDAS inactive disease (ID) versus those classified as MRI+ according to either the existing or newly proposed definitions; differences between subgroups were more notable among patients with nr-axSpA (discordant group: 42.9%; existing definition MRI+: 58.6%; newly proposed definition MRI+: 62.2%) than with r-axSpA (discordant group: 55.6%; existing definition MRI+: 60.5%; newly proposed definition MRI+: 61.0%; Figure 2).
Conclusion: In C-OPTIMISE, 11.7% fewer nr-axSpA patients would have been classified as MRI+ at baseline using the newly proposed definition versus the existing definition. Application of the preliminary MRI definition needs to be evaluated in future clinical trials to confirm its translation into fewer false-positive recruited patients.References: 1. Maksymowych WP. Rheumatology 2021;60(10):4778-4789. 2. Landewé RB. Ann Rheum Dis. 2020;79(7):920-928.
Disclosures: X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; P. Machado, AbbVie/Abbott, Eli Lilly, UCB, Novartis, Orphazyme, Galapagos; L. Bauer, UCB Pharma; B. Hoepken, UCB Pharma; M. Kim, UCB Pharma; T. Kumke, UCB Pharma; R. Tham, UCB Pharma, Veramed; M. Rudwaleit, AbbVie, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Chugai, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma.
Background/Purpose: The Assessment of SpondyloArthritis international Society (ASAS) recently proposed preliminary, more stringent, data-driven definitions for active and structural MRI lesions of the sacroiliac joint (SIJ) typical of axial spondyloarthritis (axSpA).1 We assessed clinical outcomes of patients receiving certolizumab pegol (CZP) in the C-OPTIMISE trial who were MRI-positive (MRI+) or negative (MRI-) according to both the existing and newly proposed definitions, as well as those who were MRI+ by the existing definition but MRI- by the newly proposed definition (discordant group).
Methods: C-OPTIMISE (NCT02505542) enrolled 736 patients with active axSpA who received 400 mg CZP at Weeks 0, 2, and 4, then 200mg CZP every two weeks to Week 48.2 We report: percentage of patients achieving ASAS ≥40% improvement (ASAS40) response; mean BASDAI change from baseline (CfB) and Ankylosing Spondylitis Disease Activity Score (ASDAS) disease state to Week 48. In this post hoc analysis, outcomes were stratified by axSpA subgroup (radiographic [r-]/ non-radiographic [nr]-axSpA) and MRI definition. Missing values were imputed using non-responder imputation for ASAS40 and last observation carried forward for continuous variables.
Results: Baseline MRI data were available for 657/736 (89.3%) patients, 358/657 (54.5%) of whom had r-axSpA and 299/657 (45.5%) nr-axSpA. 386/657 patients (58.8%) were classified as MRI+ according to the existing definition compared with 333/657 patients (50.7%) using the newly proposed definition; the discordant group comprised 53/657 patients (8.1%; nr-axSpA: 35/299 [11.7%]; r-axSpA: 18/358 [5.0%]; Table 1). A numerically higher proportion of newly proposed definition MRI+ patients achieved ASAS40 at Week 48 versus those fulfilling the existing MRI+ definition; patients in the discordant group responded similarly to those originally classified as MRI- (Figure 1A). Similar results were observed for mean BASDAI, with significant differences in responses between nr-axSpA patients in the newly proposed MRI+ definition and discordant groups (Figure 1B). At Week 48, a lower proportion of patients in the discordant group achieved ASDAS inactive disease (ID) versus those classified as MRI+ according to either the existing or newly proposed definitions; differences between subgroups were more notable among patients with nr-axSpA (discordant group: 42.9%; existing definition MRI+: 58.6%; newly proposed definition MRI+: 62.2%) than with r-axSpA (discordant group: 55.6%; existing definition MRI+: 60.5%; newly proposed definition MRI+: 61.0%; Figure 2).
Conclusion: In C-OPTIMISE, 11.7% fewer nr-axSpA patients would have been classified as MRI+ at baseline using the newly proposed definition versus the existing definition. Application of the preliminary MRI definition needs to be evaluated in future clinical trials to confirm its translation into fewer false-positive recruited patients.References: 1. Maksymowych WP. Rheumatology 2021;60(10):4778-4789. 2. Landewé RB. Ann Rheum Dis. 2020;79(7):920-928.



Disclosures: X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; P. Machado, AbbVie/Abbott, Eli Lilly, UCB, Novartis, Orphazyme, Galapagos; L. Bauer, UCB Pharma; B. Hoepken, UCB Pharma; M. Kim, UCB Pharma; T. Kumke, UCB Pharma; R. Tham, UCB Pharma, Veramed; M. Rudwaleit, AbbVie, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Chugai, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma.

