Back
Poster Session D
Session: (1980–2016) RA – Treatment Poster IV
2000: Tapering of Long-term, Low Dose Glucocorticoids in Senior Rheumatoid Arthritis Patients: Follow up of the Pragmatic, Multicentre, Placebo-controlled GLORIA Trial
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AA
Abdullah Almayali, BSc
Vrije Universiteit Amsterdam
Purmerend, Netherlands
Abstract Poster Presenter(s)
Abdullah Almayali1, Maarten Boers2, Linda Hartman3, Daniela OPRIS-BELINSKI4, Reinhard Bos5, Marc Kok6, Jose Pereira da Silva7, Eduard N Griep8, Ruth Klaasen9, CF Allaart10, Paul Baudoin11, Hennie Raterman12, Zoltan Szekanecz13, Frank Buttgereit14, Pavol MASARYK15, Willem Lems16, Maurizio Cutolo17 and Marieke ter Wee3, 1Amsterdam University Medical Centers, Vrije Universiteit, Purmerend, Noord-Holland, Netherlands, 2Amsterdam UMC, Vrije Universiteit, Amsterdam, Netherlands, 3Amsterdam University Medical Centers, Vrije Universiteit, Amsterdam, Netherlands, 4Carol Davila University, Bucharest, Romania, 5Medical Centre Leeuwarden, Department of Rheumatology, Leeuwarden, Netherlands, 6Department of Rheumatology and Clinical immunology, Maasstad Hospital, Rotterdam, Netherlands, 7University of Coimbra, Rheumatology, Columbia, Portugal, 8Department of Rheumatology, Antonius Hospital, Leeuwarden, Netherlands, 9Department of Rheumatology, Meander Medical Center, Amersfoort, Netherlands, 10Leiden University Medical Center, Leiden, Netherlands, 11Reumazorg Flevoland, Almere, Netherlands, 12Department of Rheumatology, Northwest Clinics, Alkmaar, Netherlands, 13Division of Rheumatology, Faculty of Medicine, Debrecen, Hungary, 14Charité Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin / DRFZ Berlin, Berlin, Germany, 15National Institute for the Rheumatic Diseases, Piešťany, Slovakia, 16Amsterdam University Medical Centers, Amsterdam, Netherlands, 17Laboratory of Experimental Rheumatology and Academic Division of Clinical Rheumatology, Department of Internal Medicine, University of Genova, IRCCS San Martino Polyclinic Hospital, Genova, Italy
Background/Purpose: Guidelines suggest glucocorticoids (GC) should be used as bridge therapy in rheumatoid arthritis (RA), but many patients are on chronic treatment, and the effects of withdrawal have not been studied extensively. The 2-year, double-blind GLORIA trial evaluated benefits and harms of low dose GC added to standard care (1). Senior RA patients (≥ 65 years) were randomly assigned to prednisolone 5 mg/day or placebo. For this study we examined disease activity, flares and signs of adrenal insufficiency after withdrawal of blinded trial medication.
Methods: After the final trial visit study medication was linearly tapered to zero in 3 months. Patients who successfully completed the trial and did not receive open-label GC during the 4 weeks after the final trial visit were included in this follow-up study.
The primary outcome was change in DAS28 at 3-month follow-up compared to the final trial visit. Secondary outcomes included the occurrence of disease flares (DAS28 increase > 0.6 or open-label GC between week 5 and 12 of the taper phase) and signs of adrenal insufficiency, assessed by 9 items selected from the 57-symptom list from the MDHAQ questionnaire and hypotension (systolic RR < 90 or diastolic RR < 60). In a subset of patients, cortisol and ACTH were measured in spot serum samples at the follow-up visit. Analysis of covariance and chi-square tests were used where appropriate, with one-sided testing for the primary outcome.
Results: 278 participants completed the GLORIA study, 21 received GC within 4 weeks after the end of the trial, 58 had missing data, leaving 199 patients. 34 patients received open label GC after 4 weeks and were excluded for the primary analysis. In the remaining 165 patients (80 prednisolone, 85 placebo), mean (SD) DAS28 was higher on placebo: 3.14 (1.04) vs 2.92 (1.13) prednisolone at the final trial visit. After tapering, disease activity increased significantly (p=0.02) in the prednisolone group to 3.18 (1.20) but was stable in placebo (3.14). The difference in the increase of DAS28 between the groups was 0.21 (95%CL –0.01, p=0.06). 44 of 99 prednisolone patients flared on tapering vs 31 of 100 placebo, relative risk 1.43 (95%CI 0.99; 2.07; p=0.07).
For signs of adrenal insufficiency, 60 prednisolone and 72 placebo patients were available (Table 1). Mean (SD) number of signs for prednisolone was 1.1 (1.1) versus 0.9 (1.3) for placebo at final trial visit and 0.8 (1.2) versus 0.8 (1.0) at follow-up. Difference in the change of the number of signs was –0.1 (95%CI –0.4;0.3; p=0.66). No differences were seen in ACTH or cortisol levels: mean (SD) ACTH was 5.8 (4.1) in 23 prednisolone patients, and 5.1 (3.7) in 24 placebo patients; cortisol 310 (166) v 296 (113), cortisol/ACTH 67 (40) v 77 (54). Two prednisolone and one placebo patient had cortisol levels below 83. None developed clinical hypoadrenalism during further follow-up.
Conclusion: Tapering prednisolone moderately increases disease activity to placebo levels (mean still at low disease activity levels) and numerically increases the risk of flare without any evidence of adrenal insufficiency. This suggests that withdrawal of low dose prednisolone is feasible after 2 years of administration.
1. Boers M. ARD 2022; doi:10.1136/annrheumdis-2021-221957
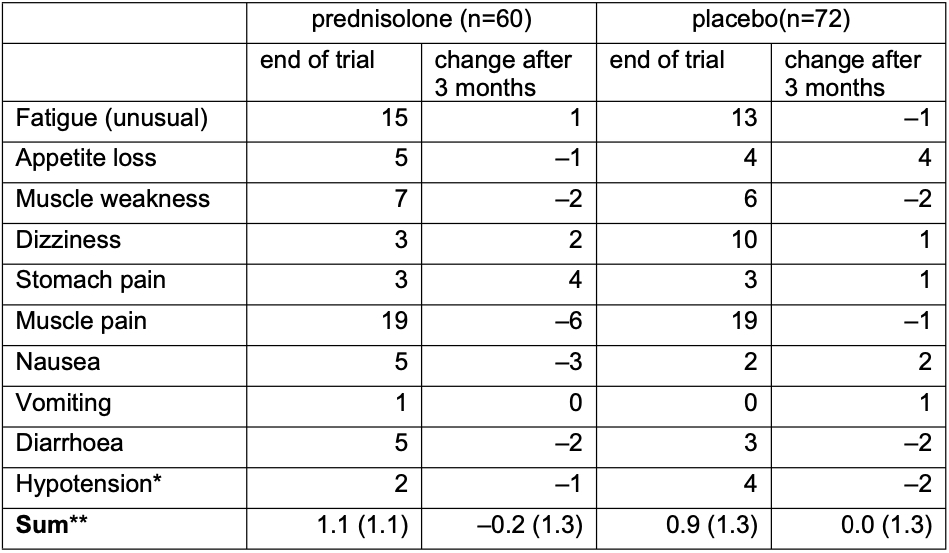 * Systolic RR < 90 or diastolic RR < 60
* Systolic RR < 90 or diastolic RR < 60
** Mean (SD)
Disclosures: A. Almayali, None; M. Boers, Novartis; L. Hartman, None; D. OPRIS-BELINSKI, AbbVie/Abbott, Pfizer, Merck/MSD, Novartis, Eli Lilly, UCB, Ewo Pharma; R. Bos, None; M. Kok, None; J. Pereira da Silva, None; E. Griep, None; R. Klaasen, None; C. Allaart, None; P. Baudoin, None; H. Raterman, AbbVie, Amgen, Celgene, Roche, Sandoz, Sanofi Genzyme, UCB; Z. Szekanecz, AbbVie, Eli Lilly, Novartis, Pfizer Inc, Roche, Sanofi, Gedeon Richter; F. Buttgereit, Horizon Therapeutics, Roche, Abbvie, AstraZeneca, Gruenenthal, Mundipharma, Pfizer; P. MASARYK, None; W. Lems, None; M. Cutolo, Bristol-Myers Squibb(BMS), Boehringer-Ingelheim, Amgen; M. ter Wee, None.
Background/Purpose: Guidelines suggest glucocorticoids (GC) should be used as bridge therapy in rheumatoid arthritis (RA), but many patients are on chronic treatment, and the effects of withdrawal have not been studied extensively. The 2-year, double-blind GLORIA trial evaluated benefits and harms of low dose GC added to standard care (1). Senior RA patients (≥ 65 years) were randomly assigned to prednisolone 5 mg/day or placebo. For this study we examined disease activity, flares and signs of adrenal insufficiency after withdrawal of blinded trial medication.
Methods: After the final trial visit study medication was linearly tapered to zero in 3 months. Patients who successfully completed the trial and did not receive open-label GC during the 4 weeks after the final trial visit were included in this follow-up study.
The primary outcome was change in DAS28 at 3-month follow-up compared to the final trial visit. Secondary outcomes included the occurrence of disease flares (DAS28 increase > 0.6 or open-label GC between week 5 and 12 of the taper phase) and signs of adrenal insufficiency, assessed by 9 items selected from the 57-symptom list from the MDHAQ questionnaire and hypotension (systolic RR < 90 or diastolic RR < 60). In a subset of patients, cortisol and ACTH were measured in spot serum samples at the follow-up visit. Analysis of covariance and chi-square tests were used where appropriate, with one-sided testing for the primary outcome.
Results: 278 participants completed the GLORIA study, 21 received GC within 4 weeks after the end of the trial, 58 had missing data, leaving 199 patients. 34 patients received open label GC after 4 weeks and were excluded for the primary analysis. In the remaining 165 patients (80 prednisolone, 85 placebo), mean (SD) DAS28 was higher on placebo: 3.14 (1.04) vs 2.92 (1.13) prednisolone at the final trial visit. After tapering, disease activity increased significantly (p=0.02) in the prednisolone group to 3.18 (1.20) but was stable in placebo (3.14). The difference in the increase of DAS28 between the groups was 0.21 (95%CL –0.01, p=0.06). 44 of 99 prednisolone patients flared on tapering vs 31 of 100 placebo, relative risk 1.43 (95%CI 0.99; 2.07; p=0.07).
For signs of adrenal insufficiency, 60 prednisolone and 72 placebo patients were available (Table 1). Mean (SD) number of signs for prednisolone was 1.1 (1.1) versus 0.9 (1.3) for placebo at final trial visit and 0.8 (1.2) versus 0.8 (1.0) at follow-up. Difference in the change of the number of signs was –0.1 (95%CI –0.4;0.3; p=0.66). No differences were seen in ACTH or cortisol levels: mean (SD) ACTH was 5.8 (4.1) in 23 prednisolone patients, and 5.1 (3.7) in 24 placebo patients; cortisol 310 (166) v 296 (113), cortisol/ACTH 67 (40) v 77 (54). Two prednisolone and one placebo patient had cortisol levels below 83. None developed clinical hypoadrenalism during further follow-up.
Conclusion: Tapering prednisolone moderately increases disease activity to placebo levels (mean still at low disease activity levels) and numerically increases the risk of flare without any evidence of adrenal insufficiency. This suggests that withdrawal of low dose prednisolone is feasible after 2 years of administration.
1. Boers M. ARD 2022; doi:10.1136/annrheumdis-2021-221957
 * Systolic RR < 90 or diastolic RR < 60
* Systolic RR < 90 or diastolic RR < 60 ** Mean (SD)
Disclosures: A. Almayali, None; M. Boers, Novartis; L. Hartman, None; D. OPRIS-BELINSKI, AbbVie/Abbott, Pfizer, Merck/MSD, Novartis, Eli Lilly, UCB, Ewo Pharma; R. Bos, None; M. Kok, None; J. Pereira da Silva, None; E. Griep, None; R. Klaasen, None; C. Allaart, None; P. Baudoin, None; H. Raterman, AbbVie, Amgen, Celgene, Roche, Sandoz, Sanofi Genzyme, UCB; Z. Szekanecz, AbbVie, Eli Lilly, Novartis, Pfizer Inc, Roche, Sanofi, Gedeon Richter; F. Buttgereit, Horizon Therapeutics, Roche, Abbvie, AstraZeneca, Gruenenthal, Mundipharma, Pfizer; P. MASARYK, None; W. Lems, None; M. Cutolo, Bristol-Myers Squibb(BMS), Boehringer-Ingelheim, Amgen; M. ter Wee, None.

