Back
Poster Session D
Session: (1950–1979) RA – Diagnosis, Manifestations, and Outcomes Poster IV
1979: Tc99m Tilmanocept Imaging Can Differentiate the Fibroid Pathotype of Rheumatoid Arthritis from Non-Fibroid Pathotypes in Patients
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- CP
Costantino Pitzalis, MD, PhD, FRCP
QMUL
Bromley Kent, United Kingdom
Abstract Poster Presenter(s)
Costantino Pitzalis1, rebecca hands2, Swamy Venuturupalli3, Ami Ben-artzi4, Eric Ruderman5, Harris Perlman6, Arthur Mandelin5, Mara Leach7, Addison Hasselbach7, Bonnie Abbruzzese7, Rachael Hershey7, Beth Potter7, Jessica Fitzpatrick7, Aaron Thornton7, Michael Blue7, Jonathan Graf8, David Ralph7 and Michael Rosol7, 1Queen Mary University of London, London, United Kingdom, 2William Harvey Research Institute, London, United Kingdom, 3Cedars-Sinai Medical Center, Los Angeles, CA, 4Ami Ben-Artzi, MD Inc., Beverly Hills, CA, 5Northwestern University Feinberg School of Medicine, Chicago, IL, 6Northwestern University, Chicago, IL, 7Navidea Biopharmaceuticals, Dublin, OH, 8Ucsf, San Francisco, CA
Background/Purpose: The primary objective of this study is to assess the relationship between joint-specific Tc99m tilmanocept (TIL) uptake values and the pathobiology of RA-involved joint tissue. The cellular composition of RA-inflamed joints is known to vary between patients and is frequently separated into one of three pathotypes: fibroid, diffuse myeloid, and lympho-myeloid. Knowledge of an individual RA patient's pathotype may be clinically important because it may predict to which RA therapy a patient is likely to respond. Imaging with TIL, a high affinity ligand to CD206 expressed on activated macrophages, offers the potential to distinguish between pathotypes without need of invasive biopsy. Our hypothesis is that the TIL imaging signal will correlate with the number and density of activated macrophages in the joints of RA patients, and that this imaging signal can provide important information about not only the disease status of the patient, but also indicate which pathotype of RA the patient has.
Methods: Eleven RA patients from a Phase 2b trial (NCT04078191) with active RA (DAS28 ≥ 3.2; ACR/EULAR 2010 Classification Criteria ≥ 6), at least one hand/wrist joint with a minimum ultrasound gray-scale synovitis score of 2 (range 0 to 3), and on stable therapy were enrolled. Hand/wrist planar gamma camera images were obtained one hour post IV administration of TIL. Quantitative image analysis was performed prior to biopsy. Images were quantitatively assessed to detect localization within synovial spaces of bilateral hands and wrists by determining average pixel intensity in each region of interest relative to average pixel intensity in an adjacent reference region, followed by comparison to a normative database of healthy control subject images. Biopsy tissues were evaluated by an expert reader using immunohistochemical staining to determine the cellular composition, including macrophage content, in the extracted inflammatory tissue. This expert reader was blinded to the imaging results prior to completing the pathology report.
Results: Image analysis conducted before the biopsies has been able to separate the subjects into at least 2 distinct and nonoverlapping classes. TIL uptake in RA-inflamed joints was able to discretely differentiate patients with the fibroid pathotype (i.e., low macrophage involvement) from those having either the diffuse myeloid or lympho-myeloid pathotypes of RA (i.e., higher macrophage involvement). Seven of the subjects had relatively low levels of TIL uptake. All seven of these subjects were found to have the fibroid pathotype. Of the remaining 4 subjects, 3 had the diffuse myeloid pathotype and 1 had the lympho-myeloid pathotype. Those subjects with either the diffuse myeloid or lympho-myeloid pathotypes had, on average, more than 3 times more TIL uptake as the average subject with the fibroid pathotype (all 4 had higher uptake).
Conclusion: These early results support the hypothesis that TIL imaging can differentiate the fibroid pathotype of rheumatoid arthritis from non-fibroid pathotypes. Further biopsy and imaging data will examine whether the diffuse myeloid vs. the lympho-myeloid pathotypes can also be discriminated.
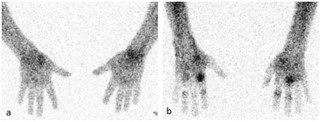 Figure 1. TIL imaging can distinguish between fibroid and non-fibroid pathotypes.
Figure 1. TIL imaging can distinguish between fibroid and non-fibroid pathotypes.
Representative images of an RA patient with the fibroid pathotype (a) and another patient with the diffuse myeloid pathotype (b). Quantification of localization of TIL in each subject demonstrates a distinct difference that is predictive of histopathology-determined pathotype.
Disclosures: C. Pitzalis, AbbVie/Abbott, Astellas, Astra-Zeneca/MedImmune, BMS, CelGene, Grunenthal, GSK, Johnson/J&J, Kiniksa, MSD, Pfizer, Sanofi, Roche/Genentech/Chugai, UCB; r. hands, None; S. Venuturupalli, Horizon Pharma USA, Inc., Kezar Life Sciences, Inc., Mallinckrodt, Inc., Navidea Biopharmaceuticals, Inc., Pfizer (Current), Bristol-Myers Squibb(BMS), Argenx, Janssen; A. Ben-artzi, None; E. Ruderman, None; H. Perlman, Janssen, kininska, exagen, LEK consultating, Guidepoint; A. Mandelin, AbbVie, Pfizer, Bristol-Myers Squibb(BMS), Horizon, CVS Caremark; M. Leach, Navidea Biopharmaceuticals, Navidea Biopharmaceuticals; A. Hasselbach, Navidea Biopharmaceuticals; B. Abbruzzese, Navidea Biopharmaceuticals; R. Hershey, Navidea Biopharmaceuticals; B. Potter, Navidea Biopharmaceuticals; J. Fitzpatrick, Navidea Biopharmaceuticals; A. Thornton, Navidea Biopharmaceuticals, Navidea Biopharmaceuticals; M. Blue, Navidea Biopharmaceuticals, Navidea Biopharmaceuticals; J. Graf, Sonoma Biotherapeutics; D. Ralph, Navidea Biopharmaceuticals; M. Rosol, Navidea Biopharmaceuticals.
Background/Purpose: The primary objective of this study is to assess the relationship between joint-specific Tc99m tilmanocept (TIL) uptake values and the pathobiology of RA-involved joint tissue. The cellular composition of RA-inflamed joints is known to vary between patients and is frequently separated into one of three pathotypes: fibroid, diffuse myeloid, and lympho-myeloid. Knowledge of an individual RA patient's pathotype may be clinically important because it may predict to which RA therapy a patient is likely to respond. Imaging with TIL, a high affinity ligand to CD206 expressed on activated macrophages, offers the potential to distinguish between pathotypes without need of invasive biopsy. Our hypothesis is that the TIL imaging signal will correlate with the number and density of activated macrophages in the joints of RA patients, and that this imaging signal can provide important information about not only the disease status of the patient, but also indicate which pathotype of RA the patient has.
Methods: Eleven RA patients from a Phase 2b trial (NCT04078191) with active RA (DAS28 ≥ 3.2; ACR/EULAR 2010 Classification Criteria ≥ 6), at least one hand/wrist joint with a minimum ultrasound gray-scale synovitis score of 2 (range 0 to 3), and on stable therapy were enrolled. Hand/wrist planar gamma camera images were obtained one hour post IV administration of TIL. Quantitative image analysis was performed prior to biopsy. Images were quantitatively assessed to detect localization within synovial spaces of bilateral hands and wrists by determining average pixel intensity in each region of interest relative to average pixel intensity in an adjacent reference region, followed by comparison to a normative database of healthy control subject images. Biopsy tissues were evaluated by an expert reader using immunohistochemical staining to determine the cellular composition, including macrophage content, in the extracted inflammatory tissue. This expert reader was blinded to the imaging results prior to completing the pathology report.
Results: Image analysis conducted before the biopsies has been able to separate the subjects into at least 2 distinct and nonoverlapping classes. TIL uptake in RA-inflamed joints was able to discretely differentiate patients with the fibroid pathotype (i.e., low macrophage involvement) from those having either the diffuse myeloid or lympho-myeloid pathotypes of RA (i.e., higher macrophage involvement). Seven of the subjects had relatively low levels of TIL uptake. All seven of these subjects were found to have the fibroid pathotype. Of the remaining 4 subjects, 3 had the diffuse myeloid pathotype and 1 had the lympho-myeloid pathotype. Those subjects with either the diffuse myeloid or lympho-myeloid pathotypes had, on average, more than 3 times more TIL uptake as the average subject with the fibroid pathotype (all 4 had higher uptake).
Conclusion: These early results support the hypothesis that TIL imaging can differentiate the fibroid pathotype of rheumatoid arthritis from non-fibroid pathotypes. Further biopsy and imaging data will examine whether the diffuse myeloid vs. the lympho-myeloid pathotypes can also be discriminated.
 Figure 1. TIL imaging can distinguish between fibroid and non-fibroid pathotypes.
Figure 1. TIL imaging can distinguish between fibroid and non-fibroid pathotypes. Representative images of an RA patient with the fibroid pathotype (a) and another patient with the diffuse myeloid pathotype (b). Quantification of localization of TIL in each subject demonstrates a distinct difference that is predictive of histopathology-determined pathotype.
Disclosures: C. Pitzalis, AbbVie/Abbott, Astellas, Astra-Zeneca/MedImmune, BMS, CelGene, Grunenthal, GSK, Johnson/J&J, Kiniksa, MSD, Pfizer, Sanofi, Roche/Genentech/Chugai, UCB; r. hands, None; S. Venuturupalli, Horizon Pharma USA, Inc., Kezar Life Sciences, Inc., Mallinckrodt, Inc., Navidea Biopharmaceuticals, Inc., Pfizer (Current), Bristol-Myers Squibb(BMS), Argenx, Janssen; A. Ben-artzi, None; E. Ruderman, None; H. Perlman, Janssen, kininska, exagen, LEK consultating, Guidepoint; A. Mandelin, AbbVie, Pfizer, Bristol-Myers Squibb(BMS), Horizon, CVS Caremark; M. Leach, Navidea Biopharmaceuticals, Navidea Biopharmaceuticals; A. Hasselbach, Navidea Biopharmaceuticals; B. Abbruzzese, Navidea Biopharmaceuticals; R. Hershey, Navidea Biopharmaceuticals; B. Potter, Navidea Biopharmaceuticals; J. Fitzpatrick, Navidea Biopharmaceuticals; A. Thornton, Navidea Biopharmaceuticals, Navidea Biopharmaceuticals; M. Blue, Navidea Biopharmaceuticals, Navidea Biopharmaceuticals; J. Graf, Sonoma Biotherapeutics; D. Ralph, Navidea Biopharmaceuticals; M. Rosol, Navidea Biopharmaceuticals.

