Back
Poster Session D
Session: (2052–2107) SLE – Diagnosis, Manifestations, and Outcomes Poster III: Outcomes
2071: The Systemic Lupus Erythematosus International Collaborating Clinics (SLICC), American College of Rheumatology (ACR), and Lupus Foundation of America (LFA) Damage Index Revision - Item Generation Phase
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- BK
Burak Kundakci, PhD, MSc
University of Manchester
Manchester, United Kingdom
Abstract Poster Presenter(s)
Burak Kundakci1, Megan R W Barber2, Ann E Clarke2, Sindhu R. Johnson3, Hermine Brunner4, Jiacai Cho5, Nathalie Costedoat-Chalumeau6, Ellen M. Ginzler7, John Hanly8, Abida Hasan7, Murat İnanç9, Naureen Kabani7, Alexandra Legge10, Kaitlin Lima11, Livia Lindoso12, Anselm Mak13, Rosalind Ramsey-Goldman11, Guillermo Ruiz-Irastorza14, Clovis A. Silva12, Farah Tamirou15, Vitor C. Trindade12, Evelyne Vinet16 and Ian N. Bruce1, 1Centre for Epidemiology Versus Arthritis, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, United Kingdom, 2University of Calgary, Division of Rheumatology, Cumming School of Medicine, Calgary, AB, Canada, 3Division of Rheumatology, Department of Medicine, Schroeder Arthritis Institute, Krembil Research Institute, Toronto Western and Mount Sinai Hospitals; Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada, 4Division of Rheumatology, Cincinnati Children's Hospital Medical Center, University of Cincinnati, Department of Pediatrics, Cincinnati, OH, 5National University Health System (NUHS), Singapore, Singapore, 6Inserm DR Paris 5, Paris, France, 7SUNY Downstate Health Sciences University, Department of Medicine, Brooklyn, NY, 8Division of Rheumatology, Queen Elizabeth II Health Sciences Center (Nova Scotia Rehabilitation Site) and Dalhousie University, Halifax, NS, Canada, 9Istanbul University Faculty of Medicine, Istanbul, Turkey, 10Arthritis Research Canada, Dalhousie University, Halifax, NS, Canada, 11Northwestern University Feinberg School of Medicine, Chicago, USA, Chicago, IL, 12Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, Brazil, 13Division of Rheumatology, Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore, 14Autoimmune Diseases Research Unit, Biocruces Bizkaia Health Research Institute, Hospital Universitario Cruces, UPV/EHU, Barakaldo, Spain, 15Rheumatology department, Cliniques Universitaires Saint-Luc, Brussels, Belgium, 16McGill University Health Centre, Montréal, QC, Canada
Background/Purpose: The SLICC, ACR and LFA embarked on a data- and expert-driven project to develop a revised systemic lupus erythematosus (SLE) organ damage index (SDI). The methodological approach includes 5 phases: updating the construct of damage (I), item generation (II), item reduction (III), item weighting and threshold determination (IV), and the assessment of validation and reliability (V). In phase I, a consensus statement was developed to define the construct of damage in SLE1. In the Item Generation phase, we aimed to develop and agree on a candidate list of items that reflect the construct of damage in SLE and are appropriate to be included in a new damage index including consideration of relevant items from adult, paediatric and young adult SLE. In this analysis, we compare the two approaches to initial item generation that were employed in a parallel process, namely a literature review and a Delphi exercise.
Methods: Item generation included a literature review and 3-part Delphi exercise. A group of lupus experts conducted a literature review to identify items that reflect the construct of damage in SLE and grouped the items into organ domains. Each domain was reviewed by paediatric rheumatologists.
Snow-ball sampling was used among SLICC members, asking them to nominate 3-4 SLE experts considering a range of clinical expertise, equality, diversity and inclusiveness factors, and the global nature of SLE research. The LFA, Lupus UK, Lupus Europe and Lupus Canada were also asked to nominate 4-6 patient/carer representatives to participate in the Delphi exercise. Participants were asked to nominate items that should be included in a revised damage index based on the updated construct definition1 using a free-text option in Delphi exercise.
Results: We established a group of 146 individuals (mean age 50.6 ranging from 28 to 79 years; 60.3% females; 58.9% white; clinical experience from 1 to 51 years) from 35 countries, broadly representative of the lupus research and patient community. There were 135 medical doctors, 2 allied health professionals and 9 patients. Of 135 medical doctors, 120 were rheumatologists, 7 internists, 5 nephrologists, 2 dermatologists, and 1 immunologist. The response rate after the first round Delphi exercise was 97.9%.
All items in the original SDI were nominated in both processes. Item generation yielded approximately 2,600 items. After rationalising for repetition, redundancy, and harmonisation of synonyms, 220 unique items were identified across 14 organ systems. The literature review proposed 4 (1.8%) unique items, 103 (46.8%) unique items were from the Delphi only and 113 (51.4%) items appeared in both exercises (Figure 1).
Conclusion: Using a combined data-driven and expert/patient-based approach, items and domains that comprise damage in SLE have been expanded. Just over half of all items were nominated by both approaches. However the Delphi exercise, that included a wide and diverse group of contributors, provided a large number of unique items for further consideration. Our data confirms the value of large group exercises early in such a process to maximise the scope of new items to consider for a revised index.
1) Johnson, S. R et al. (2021). Evaluating the construct of damage in SLE. Arthritis Care Res
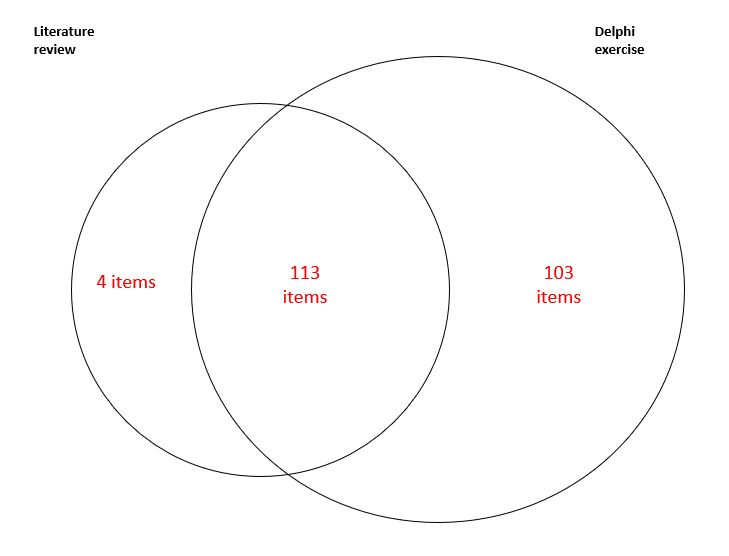 Figure 1. Number of candidate items for the revised organ damage index from literature review and the first round Delphi exercise
Figure 1. Number of candidate items for the revised organ damage index from literature review and the first round Delphi exercise
Disclosures: B. Kundakci, None; M. Barber, AstraZeneca, GlaxoSmithKline (GSK), Janssen, Sanofi-Genzyme, AbbVie; A. Clarke, AstraZeneca, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK); S. Johnson, None; H. Brunner, Cincinnati Children's Hospital, Pfizer, GlaxoSmithKlein(GSK), AbbVie/Abbott, AstraZeneca, Medimmune, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, EMD Serono, Idorsia, Cerecor, Janssen, Roche, Merck/MSD, Novartis, R-Harm, Sanofi; J. Cho, None; N. Costedoat-Chalumeau, UCB, Roche; E. Ginzler, Aurinia Pharma; J. Hanly, None; A. Hasan, None; M. İnanç, None; N. Kabani, None; A. Legge, None; K. Lima, None; L. Lindoso, None; A. Mak, None; R. Ramsey-Goldman, None; G. Ruiz-Irastorza, None; C. Silva, None; F. Tamirou, None; V. Trindade, None; E. Vinet, None; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK).
Background/Purpose: The SLICC, ACR and LFA embarked on a data- and expert-driven project to develop a revised systemic lupus erythematosus (SLE) organ damage index (SDI). The methodological approach includes 5 phases: updating the construct of damage (I), item generation (II), item reduction (III), item weighting and threshold determination (IV), and the assessment of validation and reliability (V). In phase I, a consensus statement was developed to define the construct of damage in SLE1. In the Item Generation phase, we aimed to develop and agree on a candidate list of items that reflect the construct of damage in SLE and are appropriate to be included in a new damage index including consideration of relevant items from adult, paediatric and young adult SLE. In this analysis, we compare the two approaches to initial item generation that were employed in a parallel process, namely a literature review and a Delphi exercise.
Methods: Item generation included a literature review and 3-part Delphi exercise. A group of lupus experts conducted a literature review to identify items that reflect the construct of damage in SLE and grouped the items into organ domains. Each domain was reviewed by paediatric rheumatologists.
Snow-ball sampling was used among SLICC members, asking them to nominate 3-4 SLE experts considering a range of clinical expertise, equality, diversity and inclusiveness factors, and the global nature of SLE research. The LFA, Lupus UK, Lupus Europe and Lupus Canada were also asked to nominate 4-6 patient/carer representatives to participate in the Delphi exercise. Participants were asked to nominate items that should be included in a revised damage index based on the updated construct definition1 using a free-text option in Delphi exercise.
Results: We established a group of 146 individuals (mean age 50.6 ranging from 28 to 79 years; 60.3% females; 58.9% white; clinical experience from 1 to 51 years) from 35 countries, broadly representative of the lupus research and patient community. There were 135 medical doctors, 2 allied health professionals and 9 patients. Of 135 medical doctors, 120 were rheumatologists, 7 internists, 5 nephrologists, 2 dermatologists, and 1 immunologist. The response rate after the first round Delphi exercise was 97.9%.
All items in the original SDI were nominated in both processes. Item generation yielded approximately 2,600 items. After rationalising for repetition, redundancy, and harmonisation of synonyms, 220 unique items were identified across 14 organ systems. The literature review proposed 4 (1.8%) unique items, 103 (46.8%) unique items were from the Delphi only and 113 (51.4%) items appeared in both exercises (Figure 1).
Conclusion: Using a combined data-driven and expert/patient-based approach, items and domains that comprise damage in SLE have been expanded. Just over half of all items were nominated by both approaches. However the Delphi exercise, that included a wide and diverse group of contributors, provided a large number of unique items for further consideration. Our data confirms the value of large group exercises early in such a process to maximise the scope of new items to consider for a revised index.
1) Johnson, S. R et al. (2021). Evaluating the construct of damage in SLE. Arthritis Care Res
 Figure 1. Number of candidate items for the revised organ damage index from literature review and the first round Delphi exercise
Figure 1. Number of candidate items for the revised organ damage index from literature review and the first round Delphi exerciseDisclosures: B. Kundakci, None; M. Barber, AstraZeneca, GlaxoSmithKline (GSK), Janssen, Sanofi-Genzyme, AbbVie; A. Clarke, AstraZeneca, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK); S. Johnson, None; H. Brunner, Cincinnati Children's Hospital, Pfizer, GlaxoSmithKlein(GSK), AbbVie/Abbott, AstraZeneca, Medimmune, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, EMD Serono, Idorsia, Cerecor, Janssen, Roche, Merck/MSD, Novartis, R-Harm, Sanofi; J. Cho, None; N. Costedoat-Chalumeau, UCB, Roche; E. Ginzler, Aurinia Pharma; J. Hanly, None; A. Hasan, None; M. İnanç, None; N. Kabani, None; A. Legge, None; K. Lima, None; L. Lindoso, None; A. Mak, None; R. Ramsey-Goldman, None; G. Ruiz-Irastorza, None; C. Silva, None; F. Tamirou, None; V. Trindade, None; E. Vinet, None; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK).

