Back
Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1035–1045) Spondyloarthritis Including PsA – Treatment Poster II: Mixed
1039: Differential Retention of Adalimumab and Etanercept Biosimilars Compared to Originator Treatments: Results of a Retrospective French Multicentre Study
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- GL
Guillaume LARID, MD, MSc
CHU de Poitiers
Poitiers, France
Abstract Poster Presenter(s)
Guillaume LARID1, Guy Baudens2, Alexis DANDURAND1, Pascal COQUERELLE3, Vincent Goeb4, Marie-Hélène Guyot5, Laurent Marguerie6, Frédéric MAURY7, Viellard Eric8, Eric Houvenagel9, Jean Hugues SALMON10, René-Marc Flipo11 and elisabeth gervais1, 1CHU Poitiers, Poitiers, France, 2Private practice, Valenciennes, France, 3CH Béthune, Béthune, France, 4CHU Amiens, Amiens, France, 5Private practice, Roubaix, France, 6Institut Calot, Berck-Sur-Mer, France, 7Private practice, Béthune, France, 8Private practice, St. Malo, France, 9Groupement Hospitalier de l'Institut Catholique de Lille, Lomme, France, 10Reims University Hospital, Reims, France, 11CHU Lille, Boulogne-Billancourt, France
Background/Purpose: Studies have demonstrated equivalence in term of efficacy and safety of biosimilars (bsDMARDs) compared to the originals treatments (boDMARDs) and in switching situation. Less is known about what happen when initiating a bsDMARDs in a molecule naïve patient. Objectives of our study were to compare treatment retention of subcutaneous boDMARDs and bsDMARDs globally, depending on disease (rheumatoid arthritis (RA), spondyloarthritis (SpA), or psoriatic arthritis (PsA)), molecule (etanercept (ETN) or adalimumab (ADA)), treatment's line, or presence of citrate in the context of first use of each molecule (namely initiation), and to analyse predictive factors of treatment's retention.
Methods: This multicentre retrospective study used data from shared medical records of the RIC-FRANCE network, encompassing prescription of hospital rheumatologist and attached practitioner, of patients with RA, SpA, or PsA, beginning ETN between 10/03/2016 and 07/31/2020, or ADA between 10/23/2018 and 07/31/2020. Clinical data were collected from medical records. Retention analysis was performed using Kaplan Meier curves and log-rank test. Predictive factors of retention were analysed using Cox proportional-hazard ratio. P-value < 0.05 was considered significant.
Results: 845 prescriptions were analysed: 340 boDMARDs, and 505 bsDMARDs. 57% of prescriptions concerned women, mean age was 51.8 years-old, 38% were prescriptions for RA, 16% for PsA, and 46% for SpA. An increase in the initiation over time was observed for both ETN and ADA. Retention rate of bsDMARDs was superior to boDMARDs' one (39 vs. 23 months; p=0.045). When molecules are compared, differences was significant only for ETN (45 vs. 19 months for boDMARD; p=0.0265). When diseases are compared, differences in favour of bsDMARDs was significant in RA patients only (p=0.041). Citrated treatment displayed better retention compared to citrate-free treatments (p=0.0137). Multivariable analysis of predictive factors for treatments cessation found shorter disease duration, boDMARDs prescription, hospital practitioner prescription, late line of treatment, and female sex as significant. More side effects were observed with boDMARDs especially more infections (17.8% vs. 7.8%).
Conclusion: Even if bsDMARDs' prescription increases over time, its penetration rate is still below expectations. bsDMARDs displayed better retention compared to boDMARDs, especially for ETN, and in RA patients. Citrated treatments had a better retention. Prescription by a fully hospital based rheumatologist is associated with poorer retention.
.jpg) Table 1 : Detailed characterstics of all 845 patients
Table 1 : Detailed characterstics of all 845 patients
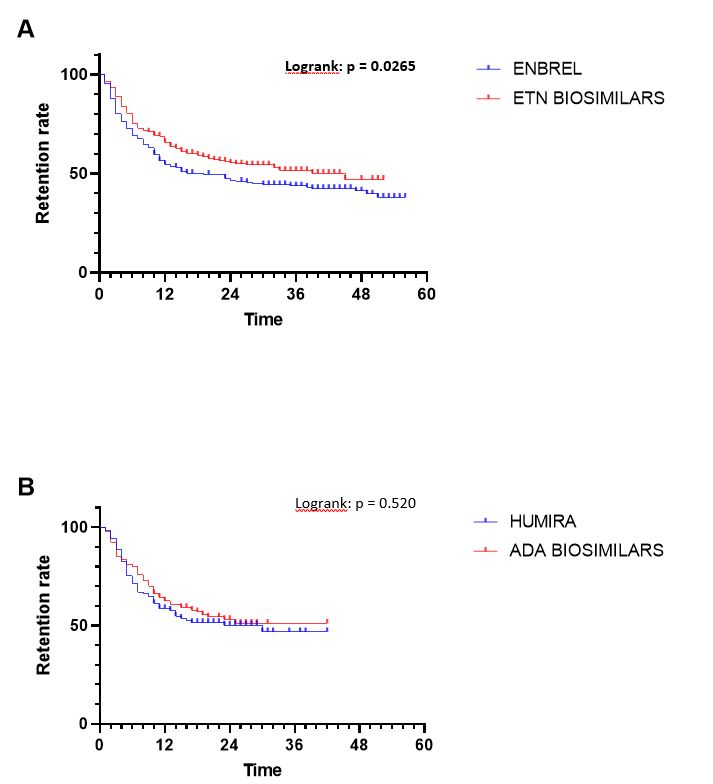 Figure 2 : Comparison of bsDMARD and boDMARD retention for ETN (A) and ADA (B)
Figure 2 : Comparison of bsDMARD and boDMARD retention for ETN (A) and ADA (B)
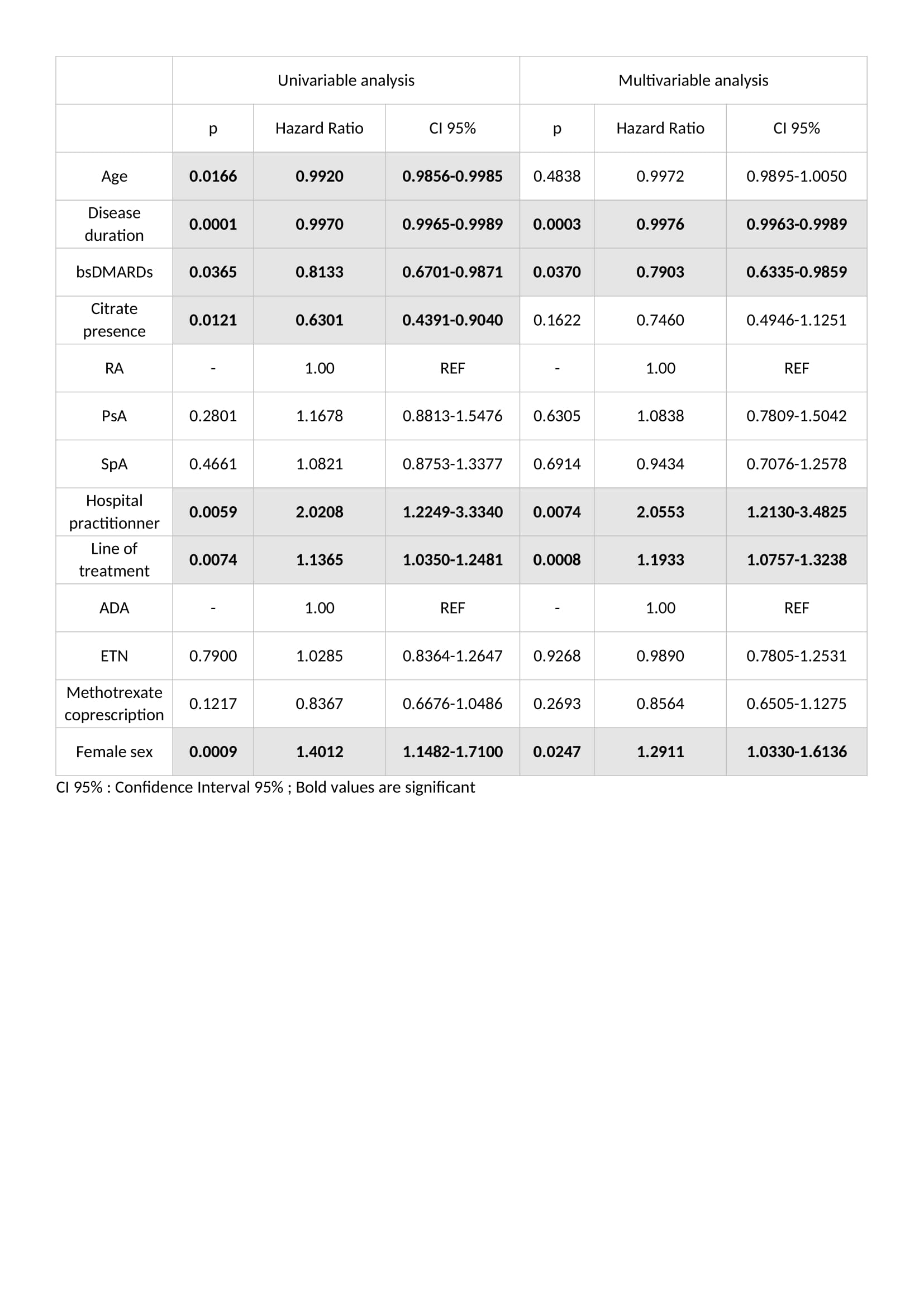 Table 2 : Predictive factors of treatment cessation in univariable and multivariable analysis
Table 2 : Predictive factors of treatment cessation in univariable and multivariable analysis
Disclosures: G. LARID, Novartis, Amgen, GlaxoSmithKlein(GSK); G. Baudens, None; A. DANDURAND, None; P. COQUERELLE, None; V. Goeb, Merck/MSD, UCB, Pfizer, Novartis, AbbVie/Abbott, Medac, Sanofi; M. Guyot, None; L. Marguerie, None; F. MAURY, None; V. Eric, None; E. Houvenagel, None; J. SALMON, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Galapagos, Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, UCB, Roche, Sanofi, Viatris; R. Flipo, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Eli Lilly, Merck/MSD, Pfizer, Roche, Sanofi; e. gervais, None.
Background/Purpose: Studies have demonstrated equivalence in term of efficacy and safety of biosimilars (bsDMARDs) compared to the originals treatments (boDMARDs) and in switching situation. Less is known about what happen when initiating a bsDMARDs in a molecule naïve patient. Objectives of our study were to compare treatment retention of subcutaneous boDMARDs and bsDMARDs globally, depending on disease (rheumatoid arthritis (RA), spondyloarthritis (SpA), or psoriatic arthritis (PsA)), molecule (etanercept (ETN) or adalimumab (ADA)), treatment's line, or presence of citrate in the context of first use of each molecule (namely initiation), and to analyse predictive factors of treatment's retention.
Methods: This multicentre retrospective study used data from shared medical records of the RIC-FRANCE network, encompassing prescription of hospital rheumatologist and attached practitioner, of patients with RA, SpA, or PsA, beginning ETN between 10/03/2016 and 07/31/2020, or ADA between 10/23/2018 and 07/31/2020. Clinical data were collected from medical records. Retention analysis was performed using Kaplan Meier curves and log-rank test. Predictive factors of retention were analysed using Cox proportional-hazard ratio. P-value < 0.05 was considered significant.
Results: 845 prescriptions were analysed: 340 boDMARDs, and 505 bsDMARDs. 57% of prescriptions concerned women, mean age was 51.8 years-old, 38% were prescriptions for RA, 16% for PsA, and 46% for SpA. An increase in the initiation over time was observed for both ETN and ADA. Retention rate of bsDMARDs was superior to boDMARDs' one (39 vs. 23 months; p=0.045). When molecules are compared, differences was significant only for ETN (45 vs. 19 months for boDMARD; p=0.0265). When diseases are compared, differences in favour of bsDMARDs was significant in RA patients only (p=0.041). Citrated treatment displayed better retention compared to citrate-free treatments (p=0.0137). Multivariable analysis of predictive factors for treatments cessation found shorter disease duration, boDMARDs prescription, hospital practitioner prescription, late line of treatment, and female sex as significant. More side effects were observed with boDMARDs especially more infections (17.8% vs. 7.8%).
Conclusion: Even if bsDMARDs' prescription increases over time, its penetration rate is still below expectations. bsDMARDs displayed better retention compared to boDMARDs, especially for ETN, and in RA patients. Citrated treatments had a better retention. Prescription by a fully hospital based rheumatologist is associated with poorer retention.
.jpg) Table 1 : Detailed characterstics of all 845 patients
Table 1 : Detailed characterstics of all 845 patients  Figure 2 : Comparison of bsDMARD and boDMARD retention for ETN (A) and ADA (B)
Figure 2 : Comparison of bsDMARD and boDMARD retention for ETN (A) and ADA (B) Table 2 : Predictive factors of treatment cessation in univariable and multivariable analysis
Table 2 : Predictive factors of treatment cessation in univariable and multivariable analysis Disclosures: G. LARID, Novartis, Amgen, GlaxoSmithKlein(GSK); G. Baudens, None; A. DANDURAND, None; P. COQUERELLE, None; V. Goeb, Merck/MSD, UCB, Pfizer, Novartis, AbbVie/Abbott, Medac, Sanofi; M. Guyot, None; L. Marguerie, None; F. MAURY, None; V. Eric, None; E. Houvenagel, None; J. SALMON, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Galapagos, Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, UCB, Roche, Sanofi, Viatris; R. Flipo, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Eli Lilly, Merck/MSD, Pfizer, Roche, Sanofi; e. gervais, None.

