Back
Poster Session D
Session: (2052–2107) SLE – Diagnosis, Manifestations, and Outcomes Poster III: Outcomes
2076: Unfavorable Outcomes Associated with Current Standard of Care in the Management of Patients with Systemic Lupus Erythematosus
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
.png)
Zahi Touma, MD, PhD
University of Toronto
Toronto, ON, Canada
Abstract Poster Presenter(s)
Zahi Touma1, Sheena Kayaniyil2, Anna Parackal2, Dennisse Bonilla1, Jiandong Su1, Christina Qian3, Sally Miller3, Shelagh Szabo3 and Shelly Chandran2, 1Schroeder Arthritis Institute, Krembil Research Institute, University Health Network and University of Toronto, Toronto, ON, Canada, 2AstraZeneca, Mississauga, ON, Canada, 3Broadstreet Health Economics & Outcomes Research, Vancouver, BC, Canada
Background/Purpose: The effectiveness of current standard of care treatment including corticosteroids (CS) in Systemic Lupus Erythematosus (SLE) is limited and has potential side-effects. Given the introduction of an SLE-approved biologic more than ten years ago, the potential for change in treatment practices related to CS use may be observed. This study examined the use of CS and the impact of CS use on irreversible organ damage in a longitudinal SLE cohort.
Methods: A retrospective observational study was conducted using data from a large SLE cohort in Canada. Adult patients (meeting ≥4 ACR SLE classification criteria, or 3 criteria and biopsy), without lupus nephritis or central nervous system lupus at entry into the cohort were included in the study. Patients were followed from index (time of entry into cohort) to last available clinic visit, with a minimum of 24 months of follow-up. Demographic and clinical characteristics including disease activity (SLEDAI-2K), treatment data and organ damage (SLICC/ACR Damage Index (SDI)) as the primary outcome stratified by CS use was assessed.
Results: A total of 1,255 patients were included (mean (standard deviation [SD]) follow-up duration of 10.5 (8.6) years, 1,111 (89%) female, and 815 (65%) White). Mean (SD) age at study entry was 35.4 (13.7) years. At index, there were 637 (51%) patients with moderate-to-severe disease activity (SLEDAI-2K ≥ 6) (Table 1). 182 (15%) patients had organ damage at index. Approximately 50% of the cohort were on antimalarials, CS, and immunosuppressants at any point during follow-up. Of those with moderate-to-severe disease activity at their last visit in the cohort, 57% were taking CS ≥ 10mg/day during their last year in the cohort. Biologic (belimumab and rituximab) use was < 3% at any point during the follow-up. Almost all patients (n=1,011, 99%) had long-term ( >6 months) cumulative CS exposure. Organ damage (SDI >0) was higher in patients with higher average CS dose and greater years of CS exposure (Table 2). The proportion of patients with any damage increased with average daily CS dose across multiple organ systems (Table 3).
Conclusion: This study in a large cohort of SLE patients shows that despite current standard of care, many SLE patients are still receiving high CS doses, especially with moderate to severe disease. High CS use was associated with irreversible organ damage. Low use of biologics was largely driven by the lack of options and lack of public access to belimumab in Canada. These findings highlight the continued unmet need in the management of SLE patients, particularly in those with moderate-to-high disease severity. Access to novel CS-sparing treatment options is critical to improve long-term outcomes for patients with SLE.
.jpg) Table 1 Patient demographics and disease characteristics at index and during follow-up
Table 1 Patient demographics and disease characteristics at index and during follow-up
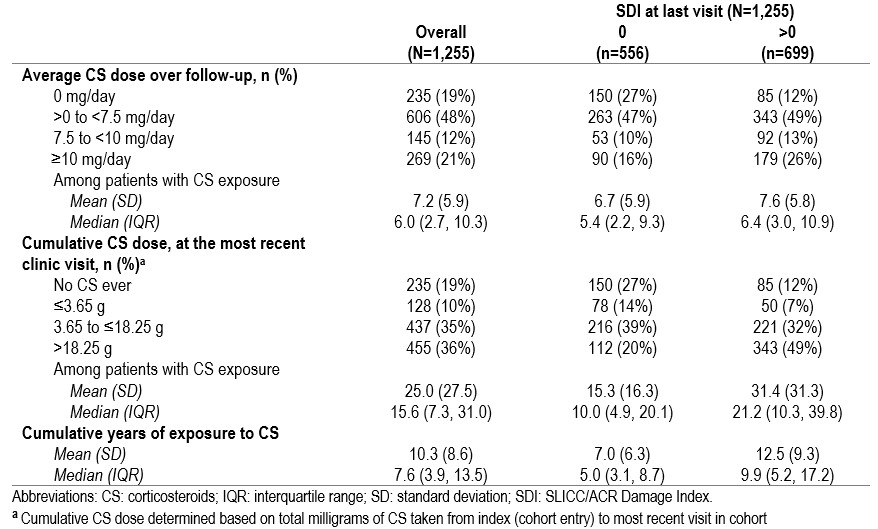 Table 2 CS overall and by organ damage (SDI)
Table 2 CS overall and by organ damage (SDI)
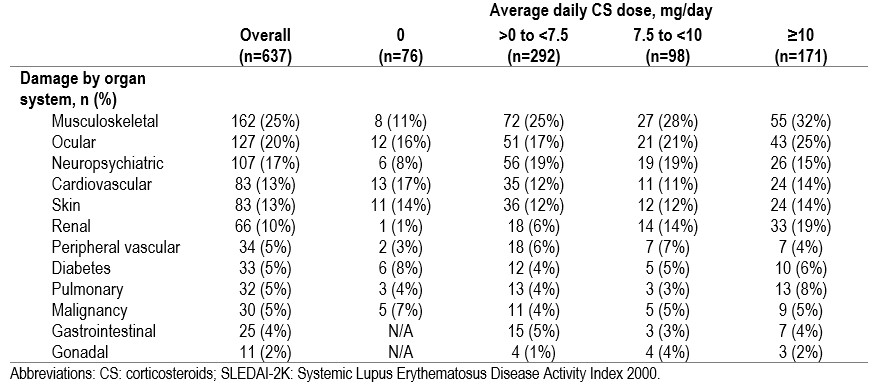 Table 3 Organ damage over follow-up stratified by average daily CS dose, in those with moderate-to-severe SLE (SLEDAI-2K ≥ 6) at baseline
Table 3 Organ damage over follow-up stratified by average daily CS dose, in those with moderate-to-severe SLE (SLEDAI-2K ≥ 6) at baseline
Disclosures: Z. Touma, None; S. Kayaniyil, AstraZeneca; A. Parackal, AstraZeneca; D. Bonilla, None; J. Su, None; C. Qian, None; S. Miller, None; S. Szabo, None; S. Chandran, AstraZeneca.
Background/Purpose: The effectiveness of current standard of care treatment including corticosteroids (CS) in Systemic Lupus Erythematosus (SLE) is limited and has potential side-effects. Given the introduction of an SLE-approved biologic more than ten years ago, the potential for change in treatment practices related to CS use may be observed. This study examined the use of CS and the impact of CS use on irreversible organ damage in a longitudinal SLE cohort.
Methods: A retrospective observational study was conducted using data from a large SLE cohort in Canada. Adult patients (meeting ≥4 ACR SLE classification criteria, or 3 criteria and biopsy), without lupus nephritis or central nervous system lupus at entry into the cohort were included in the study. Patients were followed from index (time of entry into cohort) to last available clinic visit, with a minimum of 24 months of follow-up. Demographic and clinical characteristics including disease activity (SLEDAI-2K), treatment data and organ damage (SLICC/ACR Damage Index (SDI)) as the primary outcome stratified by CS use was assessed.
Results: A total of 1,255 patients were included (mean (standard deviation [SD]) follow-up duration of 10.5 (8.6) years, 1,111 (89%) female, and 815 (65%) White). Mean (SD) age at study entry was 35.4 (13.7) years. At index, there were 637 (51%) patients with moderate-to-severe disease activity (SLEDAI-2K ≥ 6) (Table 1). 182 (15%) patients had organ damage at index. Approximately 50% of the cohort were on antimalarials, CS, and immunosuppressants at any point during follow-up. Of those with moderate-to-severe disease activity at their last visit in the cohort, 57% were taking CS ≥ 10mg/day during their last year in the cohort. Biologic (belimumab and rituximab) use was < 3% at any point during the follow-up. Almost all patients (n=1,011, 99%) had long-term ( >6 months) cumulative CS exposure. Organ damage (SDI >0) was higher in patients with higher average CS dose and greater years of CS exposure (Table 2). The proportion of patients with any damage increased with average daily CS dose across multiple organ systems (Table 3).
Conclusion: This study in a large cohort of SLE patients shows that despite current standard of care, many SLE patients are still receiving high CS doses, especially with moderate to severe disease. High CS use was associated with irreversible organ damage. Low use of biologics was largely driven by the lack of options and lack of public access to belimumab in Canada. These findings highlight the continued unmet need in the management of SLE patients, particularly in those with moderate-to-high disease severity. Access to novel CS-sparing treatment options is critical to improve long-term outcomes for patients with SLE.
.jpg) Table 1 Patient demographics and disease characteristics at index and during follow-up
Table 1 Patient demographics and disease characteristics at index and during follow-up Table 2 CS overall and by organ damage (SDI)
Table 2 CS overall and by organ damage (SDI)  Table 3 Organ damage over follow-up stratified by average daily CS dose, in those with moderate-to-severe SLE (SLEDAI-2K ≥ 6) at baseline
Table 3 Organ damage over follow-up stratified by average daily CS dose, in those with moderate-to-severe SLE (SLEDAI-2K ≥ 6) at baseline Disclosures: Z. Touma, None; S. Kayaniyil, AstraZeneca; A. Parackal, AstraZeneca; D. Bonilla, None; J. Su, None; C. Qian, None; S. Miller, None; S. Szabo, None; S. Chandran, AstraZeneca.

