Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0974–1003) SLE – Treatment Poster II
1001: Effect of Atacicept on Renal Function in Patients with Systemic Lupus Erythematosus (SLE)
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- DI
David Isenberg, MD, FRCP
University College London
London, United Kingdom
Abstract Poster Presenter(s)
David Isenberg1, Celia Lin2, Amy Kao3, Aida Aydemir4 and Caroline Gordon5, 1University College London, London, United Kingdom, 2Vera Therapeutics, Inc, Burlingame, CA, 3EMD Serono, Billerica, MA, 4EMD Serono Research & Development Institute, Inc, Billerica, MA, 5Rheumatology Research Group, Institute of Inflammation and Ageing, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom
Background/Purpose: Atacicept is a fusion protein that blocks B-lymphocyte stimulator and a proliferation-inducing ligand, which are increased in patients with SLE. APRIL-SLE was a double-blind, placebo-controlled, Phase 2 study that randomized patients with moderate-to-severe systemic lupus erythematosus (SLE) to atacicept 75 mg, atacicept 150 mg, or placebo twice-weekly for 4 weeks, then weekly for 48 weeks.The primary results of the APRIL-SLE study – the effect of atacicept compared to placebo in preventing new flares in patients with moderate-to-severe SLE – have been reported (Isenberg et al., 2013). We performed a post hoc analysis to describe the effect of atacicept compared to placebo on measures of renal function in patients with SLE; this effect has not been reported previously.
Methods: The APRIL-SLE study excluded patients with moderate to severe glomerulonephritis, as defined by either of the following: urinary protein/creatinine ratio (UPCR) >1 mg/mg and/or hematuria or a significant renal impairment as defined by estimated glomerular filtration rate (eGFR)
Results: In total, 111 patients in the placebo group, 112 patients in the atacicept 75 mg group, and 62 patients in the atacicept 150 mg group completed 52 weeks of treatment. The eGFR time course was stable for the atacicept groups compared to a 4.4% decline in the placebo group from baseline at week 52 (figure and table). UPCR from baseline at week 52 declined in the atacicept groups and increased in the placebo group.
Conclusion: Results from this double-blind, placebo-controlled, Phase 2 study suggest a potential for improved renal function with atacicept treatment of patients with moderate-to-severe SLE.
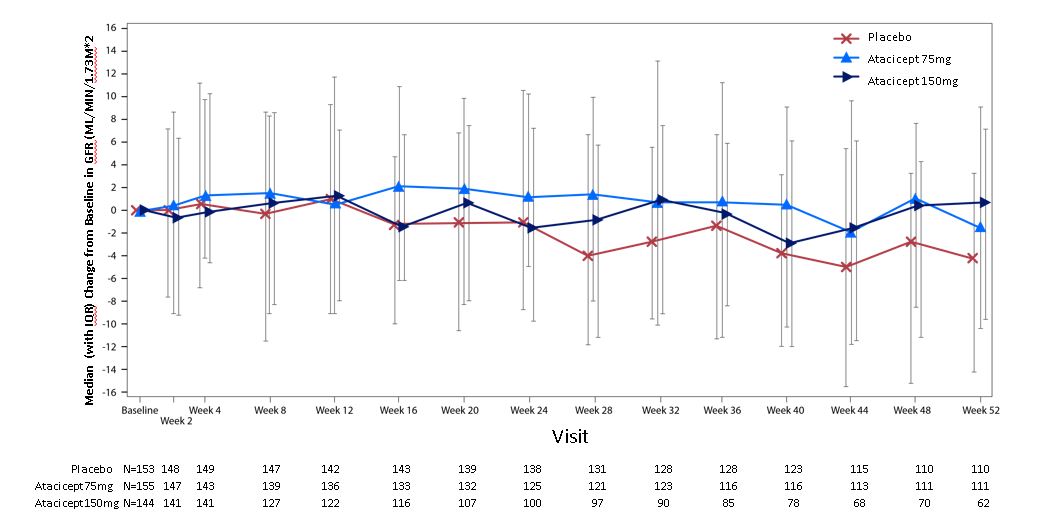 Median Change from Baseline in eGFR
Median Change from Baseline in eGFR
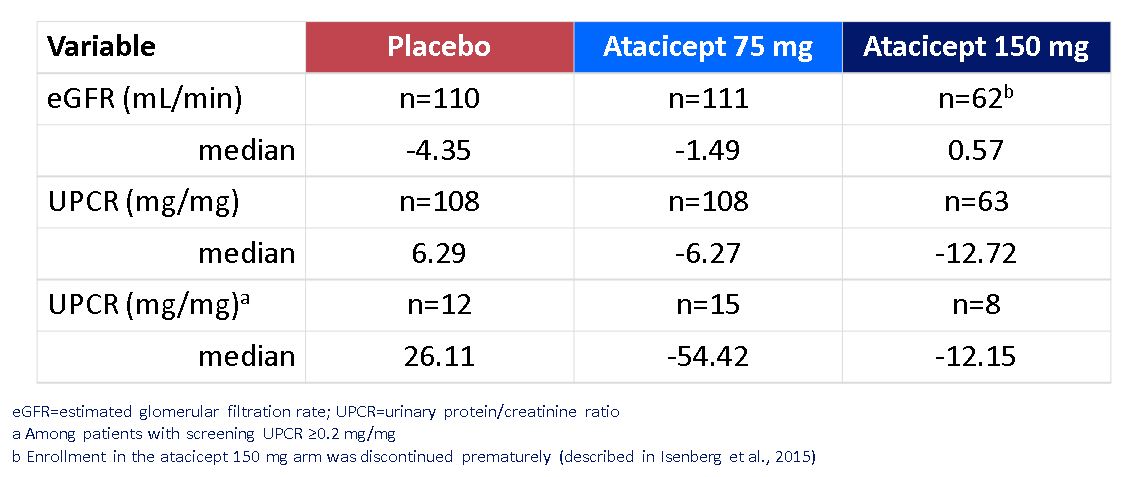 Percent Median Change in Baseline of eGFR and Proteinuria at Week 52
Percent Median Change in Baseline of eGFR and Proteinuria at Week 52
Disclosures: D. Isenberg, Merck/MSD, astra zeneca, Eli Lilly, Servier, Amgen; C. Lin, Vera Therapeutics, Inc; A. Kao, EMD Serono, Billerica, MA, USA; A. Aydemir, None; C. Gordon, UCB, Amgen, Astra-Zeneca, AbbVie, Sanofi, MGP.
Background/Purpose: Atacicept is a fusion protein that blocks B-lymphocyte stimulator and a proliferation-inducing ligand, which are increased in patients with SLE. APRIL-SLE was a double-blind, placebo-controlled, Phase 2 study that randomized patients with moderate-to-severe systemic lupus erythematosus (SLE) to atacicept 75 mg, atacicept 150 mg, or placebo twice-weekly for 4 weeks, then weekly for 48 weeks.The primary results of the APRIL-SLE study – the effect of atacicept compared to placebo in preventing new flares in patients with moderate-to-severe SLE – have been reported (Isenberg et al., 2013). We performed a post hoc analysis to describe the effect of atacicept compared to placebo on measures of renal function in patients with SLE; this effect has not been reported previously.
Methods: The APRIL-SLE study excluded patients with moderate to severe glomerulonephritis, as defined by either of the following: urinary protein/creatinine ratio (UPCR) >1 mg/mg and/or hematuria or a significant renal impairment as defined by estimated glomerular filtration rate (eGFR)
Results: In total, 111 patients in the placebo group, 112 patients in the atacicept 75 mg group, and 62 patients in the atacicept 150 mg group completed 52 weeks of treatment. The eGFR time course was stable for the atacicept groups compared to a 4.4% decline in the placebo group from baseline at week 52 (figure and table). UPCR from baseline at week 52 declined in the atacicept groups and increased in the placebo group.
Conclusion: Results from this double-blind, placebo-controlled, Phase 2 study suggest a potential for improved renal function with atacicept treatment of patients with moderate-to-severe SLE.
 Median Change from Baseline in eGFR
Median Change from Baseline in eGFR Percent Median Change in Baseline of eGFR and Proteinuria at Week 52
Percent Median Change in Baseline of eGFR and Proteinuria at Week 52Disclosures: D. Isenberg, Merck/MSD, astra zeneca, Eli Lilly, Servier, Amgen; C. Lin, Vera Therapeutics, Inc; A. Kao, EMD Serono, Billerica, MA, USA; A. Aydemir, None; C. Gordon, UCB, Amgen, Astra-Zeneca, AbbVie, Sanofi, MGP.

