Back
Poster Session D
Session: (2154–2178) Systemic Sclerosis and Related Disorders – Clinical Poster III
2170: Validation of Ranked Composite Important Difference (RCID) Score in Early Diffuse Cutaneous Systemic Sclerosis
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Francesco Del Galdo, MD, PhD
University of Leeds
Leeds, United Kingdom
Abstract Poster Presenter(s)
Francesco Del Galdo1, Suiyuan Huang2, Lesley-Anne Bissell3, sindhu johnson4, Daniel Furst5 and Dinesh Khanna6, 1Leeds Institute of Rheumatic and Musculoskeletal Medicine, Faculty of Medicine and Health and NIHR Biomedical Research Centre, University of Leeds, Leeds, United Kingdom, 2University of Michigan, Ann Arbor, MI, 3University of Leeds, Leeds, United Kingdom, 4Rheumatology, Department of Medicine, Schroeder Arthritis Institute, Toronto Western Hospital, Mount Sinai Hospital; Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, ON, Canada, 5University of California Los Angeles, Los Angeles, CA, 6Division of Rheumatology, Department of Internal Medicine, Scleroderma Program, University of Michigan, Ann Arbor, MI
Background/Purpose: The Ranked Composite Important Difference (RCID) in diffuse cutaneous Systemic Sclerosis ( dcSSc) is a clinically and patient meaningful composite score for outcome of dcSSc developed as anchor of the ACR Composite Response Index in SSc (ACR-CRISS) following OmerACT methodology. The score assigns a specific weight to Organ Failure, and Minimal Clinically important differences in FVC, mRSS and HAQ-DI both in the worsening and improving directions and it ranges from -1 ( worse possible worsening) to 1 ( best possible improvement).
Here we aimed to test the performance of the RCID in datasets from 3 randomised, placebo controlled trials in early dcSSc and determine its value in detecting worsening and improvement at group level as well as clinically meaningful anchor for the recently described revised CRISS brackets scores (1).
Methods: We analysed core set measures from 354 participants who participated in three placebo-controlled trials: Asset(2), Foccusced(3) and Fasscinate (4). We generated 10 random development datasets, selected from 2/3 of the participants, stratified by study and treatment group. The remaining participants (1/3 of the participants) formed the validation sets. Mean RCID scores of the replicate datasets were calculated for active and placebo treatments. Bootstrapping was employed to determine 95% Confidence Intervals. Worsening and improvement brackets were defined for scores within 1 STDV ( Mild worsening or improvements), between 1 and 2 STDV ( Moderate worsening or improvements) and over 2 STDV( severe worsening or marked improvement). Kappa statistics were used to analyse concordance of RCID and ACR and revised CRISS scores.
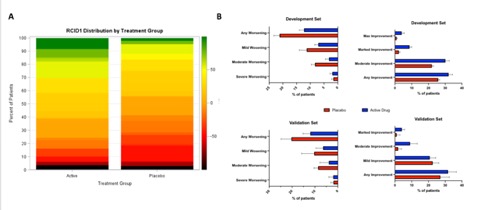
Results: RCID showed a normal distribution in the combined dataset (%Mean + STDV = 10.4 + 44.8, N=320). Positive RCID scores showed a strong correlation with ACR CRISS >0.6 with kappa statistics in development and validation sets of 0.71 (CI 0.64,0.73) and 0.71 (0.66,0.84), respectively. Accordingly, 73.1% of subjects with positive RCIDs (74% in validation set) met revised CRISS10 threshold, with 63.7 and 51.3 meeting rCRISS 20 and 30, respectively. RCID distribution by treatment group is shown in Figure 1A. Each development and validation set included 261 and 126 patients, respectively. In the development sets, 32% (C.I 29.7,34.3) of subjects on active treatment showed a positive RCID ( >0) vs 25.8 (CI: 23.3,26.6) of the placebo group. Data were confirmed in the validation set (31.7 vs 27.1). Conversely, 12.3% (10.7,14) of subjects on treatment arms showed negative RCID ( Any worsening, < 0) vs 21.1 (19.2,23.3) in the placebo groups. Proportion of patients in each RCID bracket by treatment group is shown in Figure 1B.
Conclusion: RCID, can be used as a dichotomous variable with a positive ( improving) and negative (worsening) outcome and it offers a clinically meaningful anchor to composite outcome measures like ACR CRISS or revised CRISS in early dcSSC.
Disclosures: F. Del Galdo, AbbVie/Abbott, AstraZeneca, Boehringer-Ingelheim, Mitsubishi-Tanabe, Capella biosciences, Chemomab LTD, Kymab; S. Huang, None; L. Bissell, None; s. johnson, None; D. Furst, AbbVie/Abbott, Pfizer, CORBUS, GALAPAGOS, GlaxoSmithKlein(GSK), NIH, Novartis, HORIZON, PROMETHEUS, SANOFI, Roche, Genentech, GALDERMA, CME, Bristol-Myers Squibb(BMS), Amgen; D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences.
Background/Purpose: The Ranked Composite Important Difference (RCID) in diffuse cutaneous Systemic Sclerosis ( dcSSc) is a clinically and patient meaningful composite score for outcome of dcSSc developed as anchor of the ACR Composite Response Index in SSc (ACR-CRISS) following OmerACT methodology. The score assigns a specific weight to Organ Failure, and Minimal Clinically important differences in FVC, mRSS and HAQ-DI both in the worsening and improving directions and it ranges from -1 ( worse possible worsening) to 1 ( best possible improvement).
Here we aimed to test the performance of the RCID in datasets from 3 randomised, placebo controlled trials in early dcSSc and determine its value in detecting worsening and improvement at group level as well as clinically meaningful anchor for the recently described revised CRISS brackets scores (1).
Methods: We analysed core set measures from 354 participants who participated in three placebo-controlled trials: Asset(2), Foccusced(3) and Fasscinate (4). We generated 10 random development datasets, selected from 2/3 of the participants, stratified by study and treatment group. The remaining participants (1/3 of the participants) formed the validation sets. Mean RCID scores of the replicate datasets were calculated for active and placebo treatments. Bootstrapping was employed to determine 95% Confidence Intervals. Worsening and improvement brackets were defined for scores within 1 STDV ( Mild worsening or improvements), between 1 and 2 STDV ( Moderate worsening or improvements) and over 2 STDV( severe worsening or marked improvement). Kappa statistics were used to analyse concordance of RCID and ACR and revised CRISS scores.
Results: RCID showed a normal distribution in the combined dataset (%Mean + STDV = 10.4 + 44.8, N=320). Positive RCID scores showed a strong correlation with ACR CRISS >0.6 with kappa statistics in development and validation sets of 0.71 (CI 0.64,0.73) and 0.71 (0.66,0.84), respectively. Accordingly, 73.1% of subjects with positive RCIDs (74% in validation set) met revised CRISS10 threshold, with 63.7 and 51.3 meeting rCRISS 20 and 30, respectively. RCID distribution by treatment group is shown in Figure 1A. Each development and validation set included 261 and 126 patients, respectively. In the development sets, 32% (C.I 29.7,34.3) of subjects on active treatment showed a positive RCID ( >0) vs 25.8 (CI: 23.3,26.6) of the placebo group. Data were confirmed in the validation set (31.7 vs 27.1). Conversely, 12.3% (10.7,14) of subjects on treatment arms showed negative RCID ( Any worsening, < 0) vs 21.1 (19.2,23.3) in the placebo groups. Proportion of patients in each RCID bracket by treatment group is shown in Figure 1B.
Conclusion: RCID, can be used as a dichotomous variable with a positive ( improving) and negative (worsening) outcome and it offers a clinically meaningful anchor to composite outcome measures like ACR CRISS or revised CRISS in early dcSSC.

Disclosures: F. Del Galdo, AbbVie/Abbott, AstraZeneca, Boehringer-Ingelheim, Mitsubishi-Tanabe, Capella biosciences, Chemomab LTD, Kymab; S. Huang, None; L. Bissell, None; s. johnson, None; D. Furst, AbbVie/Abbott, Pfizer, CORBUS, GALAPAGOS, GlaxoSmithKlein(GSK), NIH, Novartis, HORIZON, PROMETHEUS, SANOFI, Roche, Genentech, GALDERMA, CME, Bristol-Myers Squibb(BMS), Amgen; D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences.

