Back
Poster Session B
Epidemiology, health policy and outcomes
Session: (0695–0723) Epidemiology and Public Health Poster I
0699: Effectiveness by Disease Severity in Patients with Psoriatic Arthritis Treated with Apremilast in the CorEvitas Psoriatic Arthritis/Spondyloarthritis (PsA/SpA) Registry
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
.png)
Alexis Ogdie, MD
Rheumatologist, Associate Professor of Medicine
University of Pennsylvania
Philadelphia, PA, United States
Abstract Poster Presenter(s)
Alexis Ogdie1, Jacqueline O'Brien2, Wendi Malley2, Elizabeth Kohl2, Kate Orroth3, Yuri Klyachkin4, Myriam Cordey5 and Philip J Mease6, 1Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, 2CorEvitas, LLC, Waltham, MA, 3Amgen, Manhattan Beach, CA, 4Amgen, Lexington, KY, 5Amgen, Inc., Thousand Oaks, CA, 6Swedish Medical Center/Providence St. Joseph Health, Seattle, WA
Background/Purpose: Clinical trials of the efficacy and safety of apremilast have shown a significant therapeutic effect on disease activity across clinically diverse PsA patients. Given these favorable outcomes, apremilast represents an effective oral treatment option. Real world evidence can complement these findings in diverse populations not always studied in clinical trials. The goal of this study was to describe the effectiveness of apremilast by disease activity in a real-world population of patients with PsA.
Methods: The study included PsA patients from the CorEvitas PsA/SpA Registry initiating apremilast from March 2014 – February 2022, with Clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA) available at baseline and at 6 months. We reported patient characteristics at baseline, overall and stratified by cDAPSA category at initiation. We evaluated treatment patterns (percent who remain/discontinue therapy at or before 6 months). 6-month outcomes were reported as mean absolute differences with 95% CI (for continuous variables) and binary outcomes (e.g., treatment target achievement of cDAPSA low disease activity (LDA) or cDAPSA remission (REM)) were reported as proportions achieving the outcome with corresponding 95% CIs.
Results: 279 PsA patients initiated apremilast and had a 6-month visit. Mean age was 54.5 years and 59.8% were female. Mean duration (since diagnosis) of PsA was 6 years, 32% were systemic-naïve and 45% were biologic-naïve. Nearly half of all patients (49%) initiated apremilast as monotherapy, with the remainder initiating in combination with csDMARD (29%), b/tsDMARD (13%) or b/ts and csDMARD (10%) (Table 1). 56% remained on therapy for 6 months and 44% discontinued prior to or at the 6-month follow up. Overall, patient-reported symptoms improved over 6 months (mean improvement pain: -4.0 (-7.1, -0.9); mean improvement fatigue: -2.8 (-5.7, 0.1)), HAQ-DI remained stable over follow-up (Table 2). Among those persistent on apremilast therapy at 6 months (n=156), at the time of initiation, 18% of patients were in cDAPSA high disease activity, 41% in cDAPSA moderate disease activity, 26% in cDAPSA LDA, and 15% in cDAPSA REM (Table 3). Among persistent patients, 52% achieved treatment targets (LDA or REM), and 41% saw improvement in cDAPSA over the 6-month follow up period. Among persistent patients with moderate or high disease activity at baseline, 34% achieved LDA or REM by 6 months. Among persistent patients not in remission at initiation, 25% achieved REM by 6 months. Persistent patients in moderate disease activity were more likely to achieve LDA or REM (41%), compared to persistent patients who initiated apremilast with high disease activity (21%) (Table 3).
Conclusion: This real-world population of PsA patients achieved improved clinical and patient reported outcomes in 6 months following initiation of apremilast therapy. While cDAPSA category improvement was seen in both severe and moderate patients, persistent patients with moderate disease activity (cDAPSA) were twice as likely to achieve LDA/REM than those initiating with high disease activity.
.jpg) Table 1. Patient characteristics at time of APR initiation, for the six-month cohort, overall and by cDAPSA category at baseline
Table 1. Patient characteristics at time of APR initiation, for the six-month cohort, overall and by cDAPSA category at baseline
a Cancer = lung cancer, breast cancer, lymphoma, skin cancer (melanoma and squamous)
b IBD = Crohn’s disease, Ulcerative Colitis, Possible IBD, Other IBD
c CVD = cerebro-cardiovascular disease, consisting of: cardiac revascularization procedure (CABG, stent, angioplasty), ventricular arrhythmia, cardiac arrest, myocardial infarction, acute coronary syndrome, unstable angina, other coronary artery disease, CHF (with and without hospitalization), stroke, transient ischemic attack, other CV, deep vein thrombosis, peripheral arterial disease, pulmonary embolism, and carotid artery disease
d PsO = patients with identifying evidence of current psoriasis or a personal history of psoriasis; does not include family history of psoriasis; BSA: Limited to patients with PsO (ever)
e Axial PsA = Axial involvement for patients with PsA are defined by the following physician reported criteria: Psoriatic Arthritis (PsA) diagnosis along with any of the following a-c criteria, (a) Diagnosis of Axial Spondylarthritis (Non-radiographic or Radiographic) or Ankylosing Spondylitis, (b) Physician indicated spinal involvement or completed any of the mobility measurements (includes Occiput-to-wall distance, lateral lumbar flexion and lumbar flexion (Schöber)), (c) Selected any of the criteria for diagnosing Axial Spondyloarthritis within the Clinical Features section (includes Inflammatory back pain, ≥3 months back pain (age of onset < 45 years), Low back pain and stiffness for more than 3 months which improves with exercise by is not relieved by rest, Limitation of motion of the lumbar spine in both the sagittal and frontal planes, Active (acute) inflammation on MRI highly suggestive of sacroiliitis associated with SpA, and Sacroiliitis grade ≥ 2 bilaterally or grade 3-4 unilaterally by x-ray)
.jpg) Table 2. Continuous clinical and patient-reported outcomes - change from initiation to follow-up by cDAPSA category at baseline
Table 2. Continuous clinical and patient-reported outcomes - change from initiation to follow-up by cDAPSA category at baseline
a Limited to sample of patients with PsO (ever)
b Measured using a 100-point visual analog scale
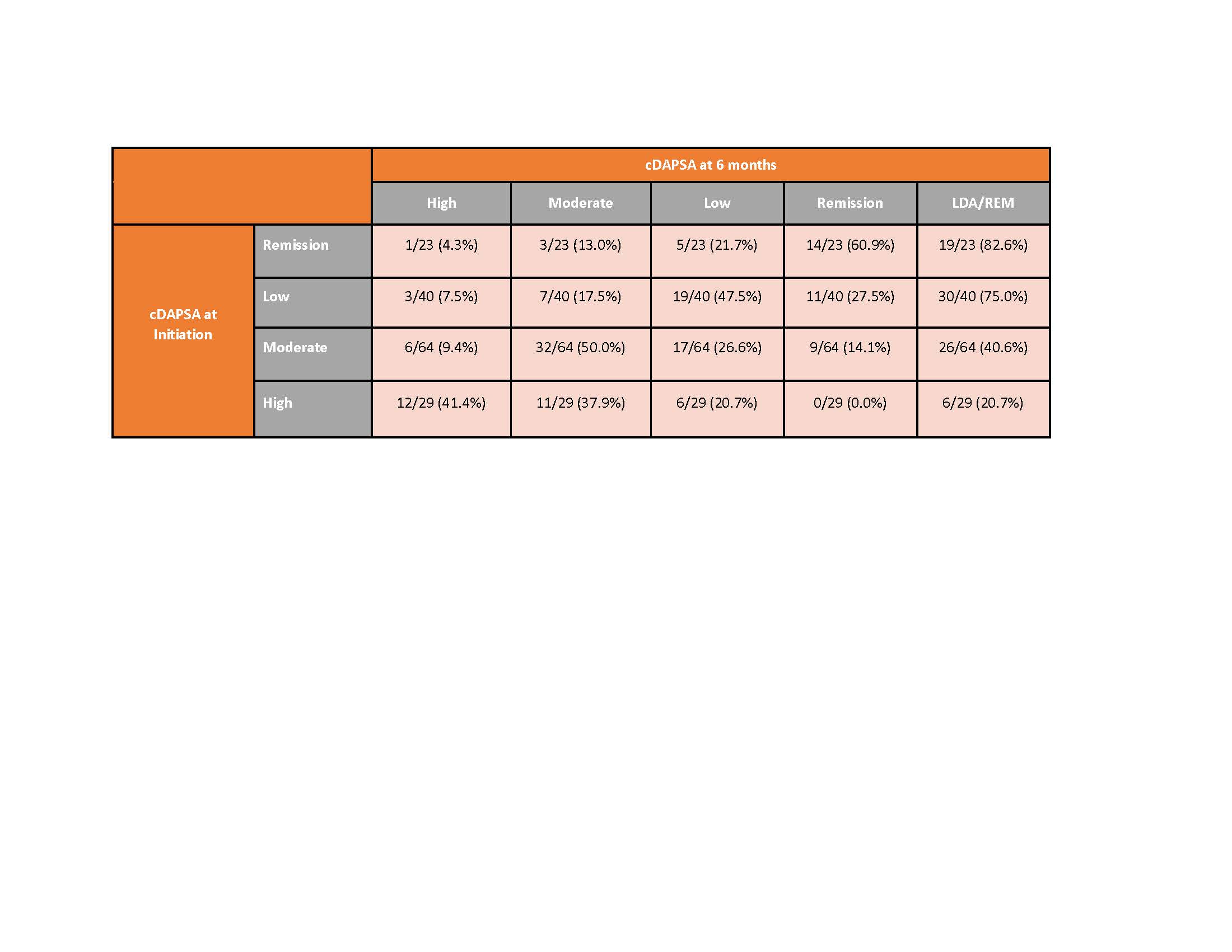 Table 3. Percent achieving cDAPSA category at six-months by baseline cDAPSA category among those persistent on apremilast at 6 months
Table 3. Percent achieving cDAPSA category at six-months by baseline cDAPSA category among those persistent on apremilast at 6 months
Disclosures: A. Ogdie, AbbVie, Amgen, Novartis, Pfizer Inc, Bristol-Myers Squibb, Celgene, Janssen, CorEvitas, Gilead Sciences, Eli Lilly, GlaxoSmithKline, Happify Health, UCB; J. O'Brien, CorEvitas, LLC.; W. Malley, None; E. Kohl, CorEvitas LLC; K. Orroth, Amgen; Y. Klyachkin, Amgen; M. Cordey, Amgen; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.
Background/Purpose: Clinical trials of the efficacy and safety of apremilast have shown a significant therapeutic effect on disease activity across clinically diverse PsA patients. Given these favorable outcomes, apremilast represents an effective oral treatment option. Real world evidence can complement these findings in diverse populations not always studied in clinical trials. The goal of this study was to describe the effectiveness of apremilast by disease activity in a real-world population of patients with PsA.
Methods: The study included PsA patients from the CorEvitas PsA/SpA Registry initiating apremilast from March 2014 – February 2022, with Clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA) available at baseline and at 6 months. We reported patient characteristics at baseline, overall and stratified by cDAPSA category at initiation. We evaluated treatment patterns (percent who remain/discontinue therapy at or before 6 months). 6-month outcomes were reported as mean absolute differences with 95% CI (for continuous variables) and binary outcomes (e.g., treatment target achievement of cDAPSA low disease activity (LDA) or cDAPSA remission (REM)) were reported as proportions achieving the outcome with corresponding 95% CIs.
Results: 279 PsA patients initiated apremilast and had a 6-month visit. Mean age was 54.5 years and 59.8% were female. Mean duration (since diagnosis) of PsA was 6 years, 32% were systemic-naïve and 45% were biologic-naïve. Nearly half of all patients (49%) initiated apremilast as monotherapy, with the remainder initiating in combination with csDMARD (29%), b/tsDMARD (13%) or b/ts and csDMARD (10%) (Table 1). 56% remained on therapy for 6 months and 44% discontinued prior to or at the 6-month follow up. Overall, patient-reported symptoms improved over 6 months (mean improvement pain: -4.0 (-7.1, -0.9); mean improvement fatigue: -2.8 (-5.7, 0.1)), HAQ-DI remained stable over follow-up (Table 2). Among those persistent on apremilast therapy at 6 months (n=156), at the time of initiation, 18% of patients were in cDAPSA high disease activity, 41% in cDAPSA moderate disease activity, 26% in cDAPSA LDA, and 15% in cDAPSA REM (Table 3). Among persistent patients, 52% achieved treatment targets (LDA or REM), and 41% saw improvement in cDAPSA over the 6-month follow up period. Among persistent patients with moderate or high disease activity at baseline, 34% achieved LDA or REM by 6 months. Among persistent patients not in remission at initiation, 25% achieved REM by 6 months. Persistent patients in moderate disease activity were more likely to achieve LDA or REM (41%), compared to persistent patients who initiated apremilast with high disease activity (21%) (Table 3).
Conclusion: This real-world population of PsA patients achieved improved clinical and patient reported outcomes in 6 months following initiation of apremilast therapy. While cDAPSA category improvement was seen in both severe and moderate patients, persistent patients with moderate disease activity (cDAPSA) were twice as likely to achieve LDA/REM than those initiating with high disease activity.
.jpg) Table 1. Patient characteristics at time of APR initiation, for the six-month cohort, overall and by cDAPSA category at baseline
Table 1. Patient characteristics at time of APR initiation, for the six-month cohort, overall and by cDAPSA category at baselinea Cancer = lung cancer, breast cancer, lymphoma, skin cancer (melanoma and squamous)
b IBD = Crohn’s disease, Ulcerative Colitis, Possible IBD, Other IBD
c CVD = cerebro-cardiovascular disease, consisting of: cardiac revascularization procedure (CABG, stent, angioplasty), ventricular arrhythmia, cardiac arrest, myocardial infarction, acute coronary syndrome, unstable angina, other coronary artery disease, CHF (with and without hospitalization), stroke, transient ischemic attack, other CV, deep vein thrombosis, peripheral arterial disease, pulmonary embolism, and carotid artery disease
d PsO = patients with identifying evidence of current psoriasis or a personal history of psoriasis; does not include family history of psoriasis; BSA: Limited to patients with PsO (ever)
e Axial PsA = Axial involvement for patients with PsA are defined by the following physician reported criteria: Psoriatic Arthritis (PsA) diagnosis along with any of the following a-c criteria, (a) Diagnosis of Axial Spondylarthritis (Non-radiographic or Radiographic) or Ankylosing Spondylitis, (b) Physician indicated spinal involvement or completed any of the mobility measurements (includes Occiput-to-wall distance, lateral lumbar flexion and lumbar flexion (Schöber)), (c) Selected any of the criteria for diagnosing Axial Spondyloarthritis within the Clinical Features section (includes Inflammatory back pain, ≥3 months back pain (age of onset < 45 years), Low back pain and stiffness for more than 3 months which improves with exercise by is not relieved by rest, Limitation of motion of the lumbar spine in both the sagittal and frontal planes, Active (acute) inflammation on MRI highly suggestive of sacroiliitis associated with SpA, and Sacroiliitis grade ≥ 2 bilaterally or grade 3-4 unilaterally by x-ray)
.jpg) Table 2. Continuous clinical and patient-reported outcomes - change from initiation to follow-up by cDAPSA category at baseline
Table 2. Continuous clinical and patient-reported outcomes - change from initiation to follow-up by cDAPSA category at baselinea Limited to sample of patients with PsO (ever)
b Measured using a 100-point visual analog scale
 Table 3. Percent achieving cDAPSA category at six-months by baseline cDAPSA category among those persistent on apremilast at 6 months
Table 3. Percent achieving cDAPSA category at six-months by baseline cDAPSA category among those persistent on apremilast at 6 monthsDisclosures: A. Ogdie, AbbVie, Amgen, Novartis, Pfizer Inc, Bristol-Myers Squibb, Celgene, Janssen, CorEvitas, Gilead Sciences, Eli Lilly, GlaxoSmithKline, Happify Health, UCB; J. O'Brien, CorEvitas, LLC.; W. Malley, None; E. Kohl, CorEvitas LLC; K. Orroth, Amgen; Y. Klyachkin, Amgen; M. Cordey, Amgen; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.

