Back
Ignite Talk
Session: Ignite Session 7A
2086: Remission and Low Disease Activity Are Associated with Lower Health Care Costs in an International Inception Cohort of Patients with Systemic Lupus Erythematosus
Monday, November 14, 2022
1:10 PM – 1:15 PM Eastern Time
Location: Northern Liberties Stage
- AC
Ann Clarke, MD, MSc
University of Calgary
Calgary, AB, CanadaDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
Ann E Clarke1, Manuel Ugarte-Gil2, Megan Barber3, John Hanly4, Murray Urowitz5, Yvan St. Pierre6, Caroline Gordon7, Sang-Cheol Bae8, Juanita Romero-Diaz9, Jorge Sanchez-Guerrero10, Sasha Bernatsky6, Daniel Wallace11, David Isenberg12, Anisur Rahman13, Joan Merrill14, Paul R Fortin15, Dafna Gladman16, Ian N. Bruce17, Michelle Petri18, Ellen M. Ginzler19, Mary Anne Dooley20, Rosalind Ramsey-Goldman21, Susan Manzi22, Andreas Jönsen23, Ronald van Vollenhoven24, Cynthia Aranow25, Meggan Mackay25, Guillermo Ruiz-Irastorza26, S. Sam Lim27, Murat Inanc28, Kenneth Kalunian29, Soren Jacobsen30, Christine Peschken31, Diane Kamen32, Anca Askanase33, Bernardo Pons-Estel34 and Graciela Alarcón35, 1University of Calgary, Division of Rheumatology, Cumming School of Medicine, Calgary, AB, Canada, 2Universidad Cientifica del Sur/Hospital Guillermo Almenara Irigoyen. EsSalud, Lima, Peru, 3Cumming School of Medicine, University of Calgary, Calgary, AB, Canada, 4Division of Rheumatology, Queen Elizabeth II Health Sciences Center (Nova Scotia Rehabilitation Site) and Dalhousie University, Halifax, NS, Canada, 5University of Toronto, University Health Network, Schroeder Arthritis Institute, Toronto, ON, Canada, 6Research Institute of the McGill University Health Centre, Montréal, QC, Canada, 7Rheumatology Research Group, Institute of Inflammation and Ageing, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom, 8Hanyang University Medical Center, Seoul, Republic of Korea, 9Instituto Nacional de Ciencias Medicas y Nutricion SZ, Ciudad de México, Mexico, 10Mount Sinai Hospital and University Health Network, University of Toronto, Toronto, ON, Canada, 11Cedars-Sinai Medical Center, Los Angeles, CA, 12University College London, London, United Kingdom, 13Centre for Rheumatology, Department of Medicine, University College London, London, United Kingdom, 14Oklahoma Medical Research Foundation, Oklahoma City, OK, 15Centre ARThrite - CHU de Québec - Université Laval, Québec, QC, Canada, 16Toronto Western Hospital, Schroeder Arthritis Institute, Toronto, ON, Canada, 17Centre for Epidemiology Versus Arthritis, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, United Kingdom, 18Johns Hopkins University School of Medicine, Division of Rheumatology, Baltimore, MD, 19SUNY Downstate Health Sciences University, Department of Medicine, Brooklyn, NY, 20Raleigh Neurology Associates, Chapel Hill, NC, 21Northwestern University Feinberg School of Medicine, Chicago, USA, Chicago, IL, 22Allegheny Health Network, Lupus Center of Excellence, Wexford, PA, 23Department of Clinical Sciences, Lund, Section for Rheumatology, Lund University, Lund and Skåne University Hospital, Lund, Sweden, 24Amsterdam University Medical Centers, Amsterdam, Netherlands, 25Feinstein Institutes for Medical Research, Manhasset, NY, 26Autoimmune Diseases Research Unit, Biocruces Bizkaia Health Research Institute, Hospital Universitario Cruces, UPV/EHU, Barakaldo, Spain, 27Emory University, Atlanta, GA, 28Division of Rheumatology, Department of Internal Medicine, Istanbul Medical Faculty, Istanbul University, Istambul, Turkey, 29University of California San Diego, La Jolla, CA, 30Rigshospitalet, Copenhagen, Denmark, 31University of Manitoba, Winnipeg, MB, Canada, 32Medical University of South Carolina, Charleston, SC, 33Columbia University Medical Center, New York, NY, 34Grupo Oroño - Centro Regional de Enfermedades Autoinmunes y Reumáticas (GO-CREAR), Rosario, Argentina, 35The University of Alabama at Birmingham, Oakland
Background/Purpose: Remission and low disease activity (LDA) are associated with decreased flares, damage, and mortality. However, little is known about the impact of disease activity states (DAS) on health care costs. We determined the independent impact of different definitions of remission and LDA on direct and indirect costs (DC, IC) in a multicentre, multi-ethnic inception cohort.
Methods: Patients fulfilling revised ACR classification criteria for SLE from 33 centres in 11 countries were enrolled within 15 months of diagnosis and assessed annually. Patients with ≥2 annual assessments were included. Five mutually independent DAS were defined:
1) Remission off-treatment: clinical (c) SLEDAI-2K=0, without prednisone or immunosuppressants
2) Remission on-treatment: cSLEDAI-2K=0, prednisone ≤5mg/d and/or maintenance immunosuppressants
3) LDA-Toronto Cohort (TC): cSLEDAI-2K≤2, without prednisone or immunosuppressants
4) Modified Lupus LDA State (mLLDAS): SLEDAI-2K≤4, no activity in major organs/systems, no new disease activity, prednisone ≤7.5mg/d and/or maintenance immunosuppressants
5) Active: all remaining assessments
Antimalarials were permitted in all DAS. At each assessment, patients were stratified into 1 DAS; if >1 definition was fulfilled per assessment, the patient was stratified into the most stringent. The proportion of time patients were in a specific DAS at each assessment since cohort entry was determined.
At each assessment, annual DC and IC were based on health resource use and lost workforce/non-workforce productivity over the preceding year. Resource use was costed using 2021 Canadian prices and lost productivity using Statistics Canada age-and-sex-matched wages.
To examine the association between the proportion of time in a specific DAS at each assessment since cohort entry and annual DC and IC, multivariable random-effects linear regression modelling was used. Potential covariates included age at diagnosis, disease duration, sex, race/ethnicity, education, region, smoking, and alcohol use.
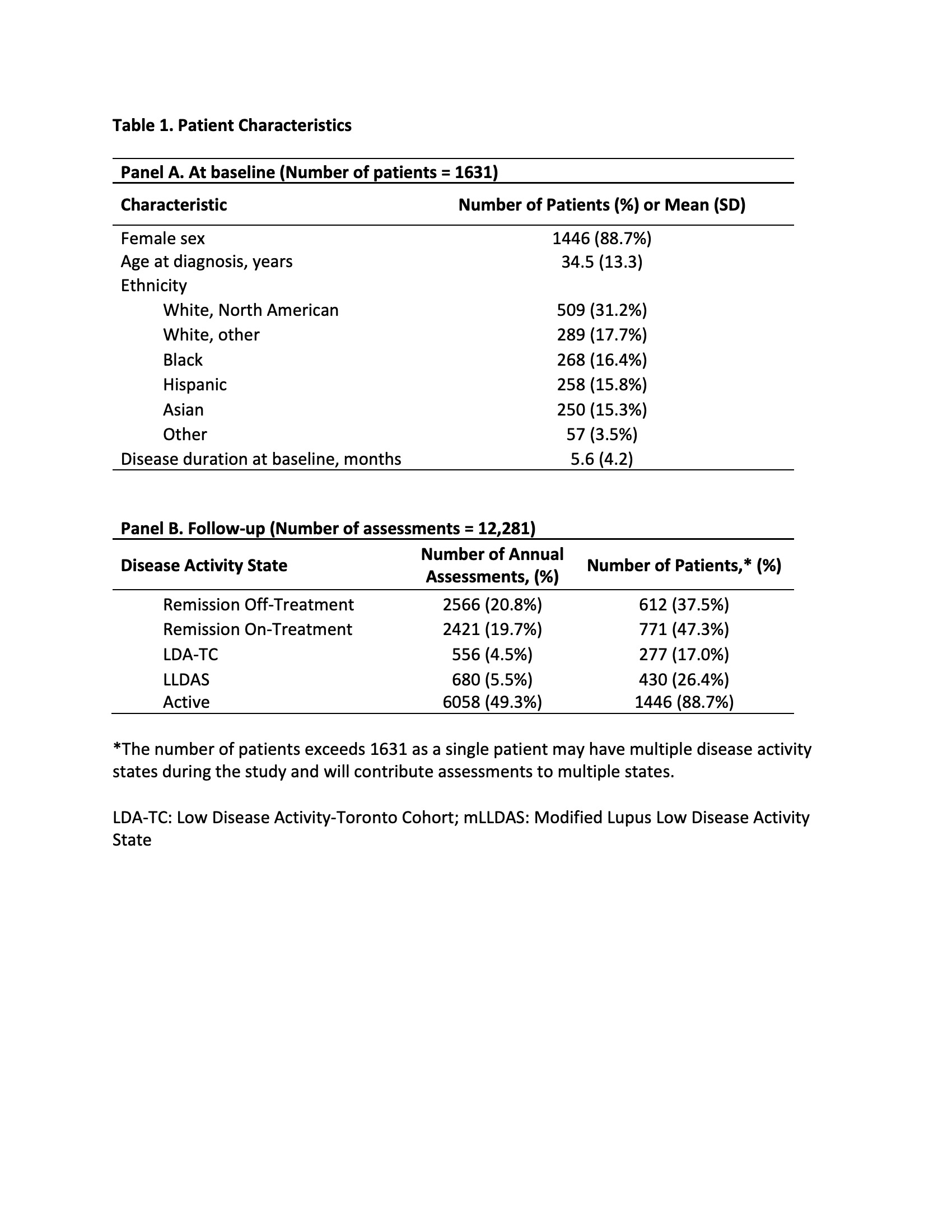
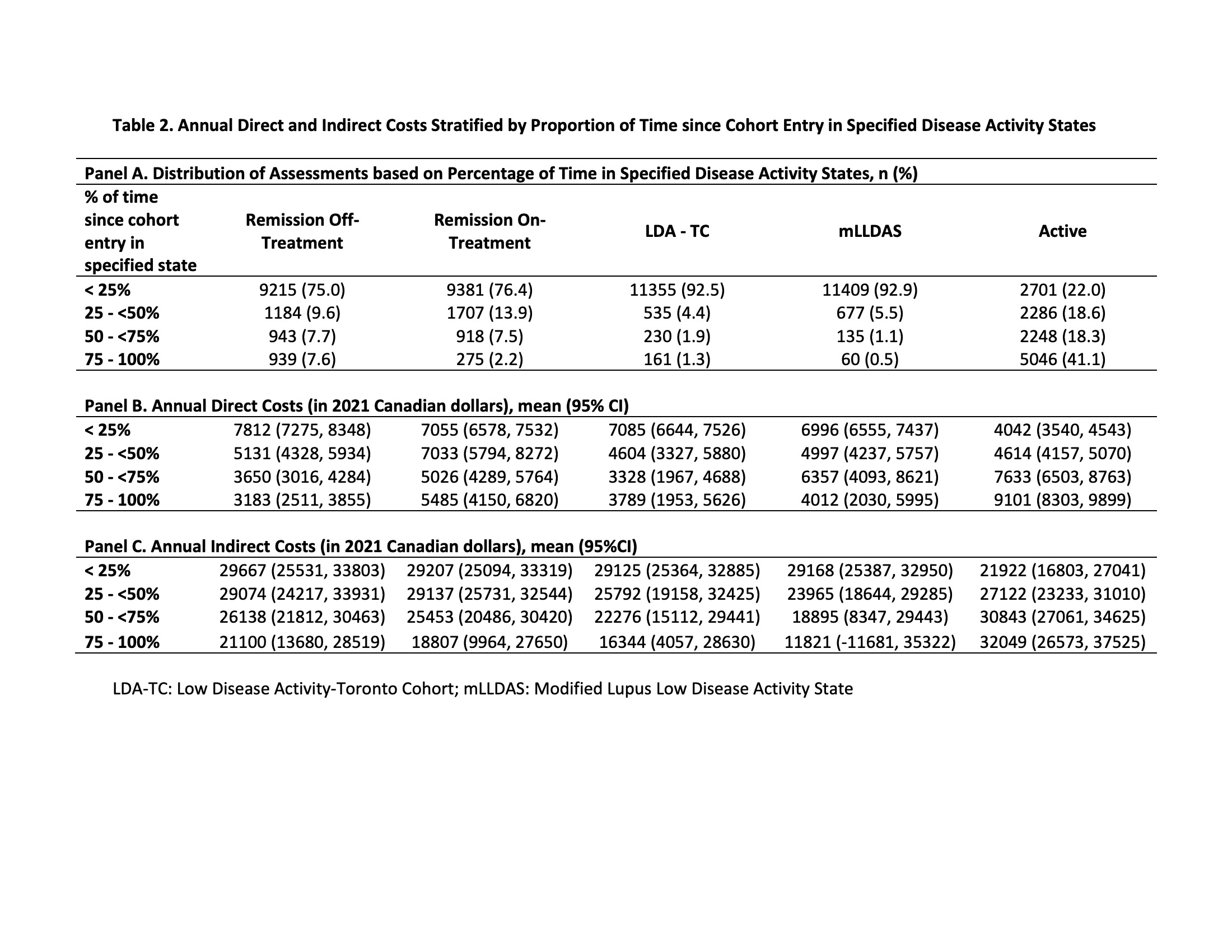
Results: 1631 patients (88.7% female, 48.9% White, mean age at diagnosis 34.5) were followed for a mean of 7.7 (SD 4.7) years. Across 12,281 assessments, 49.3% were classified as active (Table 1, Panel B). Patients spending < 25% vs 75-100% of their time since cohort entry in an active DAS had lower annual DC and IC (DC $4042 vs $9101, difference -$5060, 95%CI -$5983, -$4136; IC $21,922 vs $32,049, difference -$10,127, 95%CI -$16,754, -$3499) (Table 2, Panel B&C).
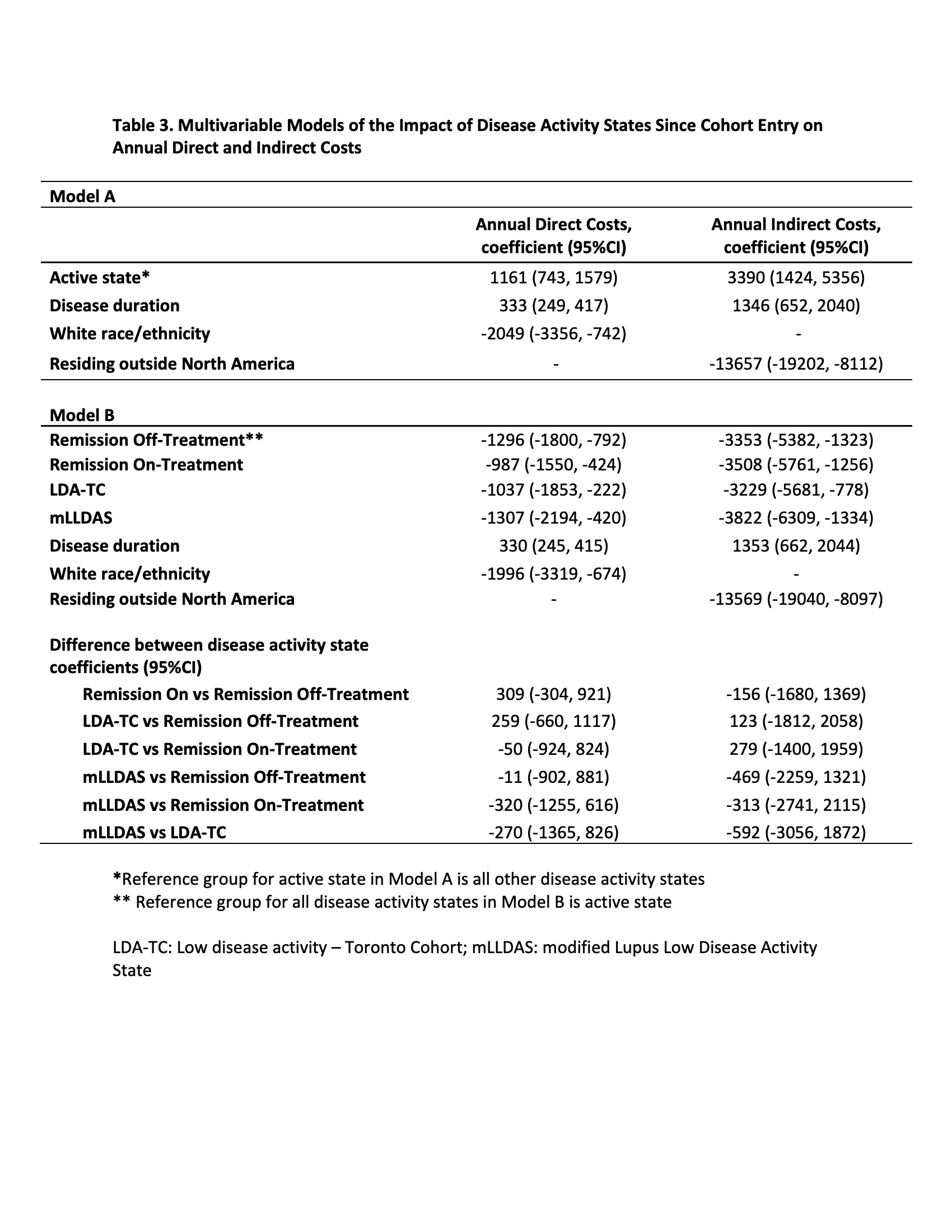
In multivariable models, remission and LDA (per 25% increase in time spent in specified DAS versus active) were associated with lower annual DC and IC: remission off-treatment (DC -$1296, 95%CI -$1800, -$792; IC -$3353, 95%CI -$5382, -$1323), remission on-treatment (DC -$987, 95%CI -$1550, -$424; IC -$3508, 95%CI -$5761, -$1256), LDA-TC (DC -$1037, 95%CI -$1853, -$222; IC -$3229, 95%CI -$5681, -$778) and mLLDAS (DC -$1307, 95%CI -$2194, -$420; IC -$3822, 95%CI -$6309, $-1334) (Table 3, Model B). There were no differences in costs between remission and LDA.
Conclusion: Remission and LDA are associated with lower costs, likely mediated through the known association of these DAS with more favourable clinical outcomes.
Disclosures: A. Clarke, AstraZeneca, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK); M. Ugarte-Gil, Janssen, Pfizer; M. Barber, Sanofi-Genzyme, AbbVie/Abbott, GlaxoSmithKlein(GSK), AstraZeneca, Janssen; J. Hanly, None; M. Urowitz, None; Y. St. Pierre, None; C. Gordon, UCB, Amgen, Astra-Zeneca, AbbVie, Sanofi, MGP; S. Bae, None; J. Romero-Diaz, Biogen; J. Sanchez-Guerrero, None; S. Bernatsky, None; D. Wallace, None; D. Isenberg, Merck/MSD, astra zeneca, Eli Lilly, Servier, Amgen; A. Rahman, None; J. Merrill, UCB, GlaxoSmithKline, AbbVie, EMD Serono, Remegen, Celgene/Bristol Myers Squibb, AstraZeneca, Amgen, Janssen, Lilly, Genentech, Aurinia, Astellas, Alexion, Sanofi, Zenas, Proventio; P. Fortin, AstraZeneca, GlaxoSmithKlein(GSK); D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; E. Ginzler, Aurinia Pharma; M. Dooley, None; R. Ramsey-Goldman, None; S. Manzi, AstraZeneca, GlaxoSmithKline (GSK), Exagen Diagnostics Inc, AbbVie, HGS, Cugene, Lilly, UCB Advisory Board, Lupus Foundation of America; A. Jönsen, None; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; C. Aranow, None; M. Mackay, None; G. Ruiz-Irastorza, None; S. Lim, None; M. Inanc, None; K. Kalunian, AbbVie/Abbott, Amgen, AstraZeneca, Aurinia, Biogen, Bristol Myers Squibb (BMS), Eli Lilly, Equillium, Genentech, Gilead, Janssen, Roche, Lupus Research Alliance, Pfizer, Sanford Consortium, Viela, Nektar; S. Jacobsen, None; C. Peschken, None; D. Kamen, None; A. Askanase, AstraZeneca, GlaxoSmithKlein(GSK), Aurinia, Amgen, Pfizer, Idorsia, Eli Lilly, UCB, AbbVie/Abbott, Janssen, Bristol-Myers Squibb(BMS); B. Pons-Estel, None; G. Alarcón, None.
Background/Purpose: Remission and low disease activity (LDA) are associated with decreased flares, damage, and mortality. However, little is known about the impact of disease activity states (DAS) on health care costs. We determined the independent impact of different definitions of remission and LDA on direct and indirect costs (DC, IC) in a multicentre, multi-ethnic inception cohort.
Methods: Patients fulfilling revised ACR classification criteria for SLE from 33 centres in 11 countries were enrolled within 15 months of diagnosis and assessed annually. Patients with ≥2 annual assessments were included. Five mutually independent DAS were defined:
1) Remission off-treatment: clinical (c) SLEDAI-2K=0, without prednisone or immunosuppressants
2) Remission on-treatment: cSLEDAI-2K=0, prednisone ≤5mg/d and/or maintenance immunosuppressants
3) LDA-Toronto Cohort (TC): cSLEDAI-2K≤2, without prednisone or immunosuppressants
4) Modified Lupus LDA State (mLLDAS): SLEDAI-2K≤4, no activity in major organs/systems, no new disease activity, prednisone ≤7.5mg/d and/or maintenance immunosuppressants
5) Active: all remaining assessments
Antimalarials were permitted in all DAS. At each assessment, patients were stratified into 1 DAS; if >1 definition was fulfilled per assessment, the patient was stratified into the most stringent. The proportion of time patients were in a specific DAS at each assessment since cohort entry was determined.
At each assessment, annual DC and IC were based on health resource use and lost workforce/non-workforce productivity over the preceding year. Resource use was costed using 2021 Canadian prices and lost productivity using Statistics Canada age-and-sex-matched wages.
To examine the association between the proportion of time in a specific DAS at each assessment since cohort entry and annual DC and IC, multivariable random-effects linear regression modelling was used. Potential covariates included age at diagnosis, disease duration, sex, race/ethnicity, education, region, smoking, and alcohol use.
Results: 1631 patients (88.7% female, 48.9% White, mean age at diagnosis 34.5) were followed for a mean of 7.7 (SD 4.7) years. Across 12,281 assessments, 49.3% were classified as active (Table 1, Panel B). Patients spending < 25% vs 75-100% of their time since cohort entry in an active DAS had lower annual DC and IC (DC $4042 vs $9101, difference -$5060, 95%CI -$5983, -$4136; IC $21,922 vs $32,049, difference -$10,127, 95%CI -$16,754, -$3499) (Table 2, Panel B&C).
In multivariable models, remission and LDA (per 25% increase in time spent in specified DAS versus active) were associated with lower annual DC and IC: remission off-treatment (DC -$1296, 95%CI -$1800, -$792; IC -$3353, 95%CI -$5382, -$1323), remission on-treatment (DC -$987, 95%CI -$1550, -$424; IC -$3508, 95%CI -$5761, -$1256), LDA-TC (DC -$1037, 95%CI -$1853, -$222; IC -$3229, 95%CI -$5681, -$778) and mLLDAS (DC -$1307, 95%CI -$2194, -$420; IC -$3822, 95%CI -$6309, $-1334) (Table 3, Model B). There were no differences in costs between remission and LDA.
Conclusion: Remission and LDA are associated with lower costs, likely mediated through the known association of these DAS with more favourable clinical outcomes.



Disclosures: A. Clarke, AstraZeneca, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK); M. Ugarte-Gil, Janssen, Pfizer; M. Barber, Sanofi-Genzyme, AbbVie/Abbott, GlaxoSmithKlein(GSK), AstraZeneca, Janssen; J. Hanly, None; M. Urowitz, None; Y. St. Pierre, None; C. Gordon, UCB, Amgen, Astra-Zeneca, AbbVie, Sanofi, MGP; S. Bae, None; J. Romero-Diaz, Biogen; J. Sanchez-Guerrero, None; S. Bernatsky, None; D. Wallace, None; D. Isenberg, Merck/MSD, astra zeneca, Eli Lilly, Servier, Amgen; A. Rahman, None; J. Merrill, UCB, GlaxoSmithKline, AbbVie, EMD Serono, Remegen, Celgene/Bristol Myers Squibb, AstraZeneca, Amgen, Janssen, Lilly, Genentech, Aurinia, Astellas, Alexion, Sanofi, Zenas, Proventio; P. Fortin, AstraZeneca, GlaxoSmithKlein(GSK); D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; E. Ginzler, Aurinia Pharma; M. Dooley, None; R. Ramsey-Goldman, None; S. Manzi, AstraZeneca, GlaxoSmithKline (GSK), Exagen Diagnostics Inc, AbbVie, HGS, Cugene, Lilly, UCB Advisory Board, Lupus Foundation of America; A. Jönsen, None; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; C. Aranow, None; M. Mackay, None; G. Ruiz-Irastorza, None; S. Lim, None; M. Inanc, None; K. Kalunian, AbbVie/Abbott, Amgen, AstraZeneca, Aurinia, Biogen, Bristol Myers Squibb (BMS), Eli Lilly, Equillium, Genentech, Gilead, Janssen, Roche, Lupus Research Alliance, Pfizer, Sanford Consortium, Viela, Nektar; S. Jacobsen, None; C. Peschken, None; D. Kamen, None; A. Askanase, AstraZeneca, GlaxoSmithKlein(GSK), Aurinia, Amgen, Pfizer, Idorsia, Eli Lilly, UCB, AbbVie/Abbott, Janssen, Bristol-Myers Squibb(BMS); B. Pons-Estel, None; G. Alarcón, None.

