Back
Ignite Talk
Session: Ignite Session 7B
0288: Comparison of the Effect of Different Janus Kinase Inhibitors on Activation, Function and Property of NK Cells to Control Cancer Cell Lines Proliferation: An Ex Vivo and in Vitro Study
Monday, November 14, 2022
1:25 PM – 1:30 PM Eastern Time
Location: Center City Stage
- LM
Loïc Meudec, MD
Université Paris-Saclay
Le Kremlin-Bicêtre, FranceDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
Loïc Meudec1, Pauline Richebé1, Juliette Pascaud2, Xavier Mariette3 and Gaetane Nocturne4, 1Université Paris-Saclay, AP-HP-Hôpital Bicêtre, Le Kremlin Bicêtre, France, 2Université Paris-Saclay, INSERM U1184, Le Kremlin Bicêtre, France, 3Paris-Saclay University, Rueil Malmaison, Ile-de-France, France, 4APHP, Le Kremlin Bicêtre, France
Background/Purpose: Janus kinase inhibitors (JAKi) are effective treatments licensed in rheumatoid arthritis (RA). Concerns about a risk of cancer may arise with JAKi as in every immunosuppression situation. Recently the ORAL Surveillance trial comparing tofacitinib (TOFA) to TNF inhibitors (TNFi) in RA patients over fifty years with at least one cardiovascular risk highlighted an increased risk of cancer especially lung cancer and lymphoma1. Given their role in the antitumor surveillance and antiviral response, especially against herpes virus that have been shown to be increased with JAKi, NK cells may play a causal role in these adverse events.
Our hypothesis is that JAKi may promote tumorigenesis by inhibiting NK cells and that this impact could vary depending of the selectivity profile of these drugs. Thus our objective was to assess the phenotypic and functional impact of JAKi on NK cells.
Methods: We first performed ex vivo phenotypic analyses of NK cells in RA patients treated with TOFA or baricitinib (BARI) compared to patients treated with methotrexate (MTX). To go deeper, we exposed in vitro sorted NK cells from healthy donors to the 4 JAKi [(TOFA, BARI, upadacitinib (UPA) and filgotinib (FILGO)] or control (DMSO) for 6 days. We used 3 different doses carefully chosen to mimic in vivo exposure: we first assess the in vitro dose corresponding to the mean dose in patients at licensed dose [therapeutic concentration (therap)], then we framed this dose with a lower dose (infra, therap -50%) and supra dose (supra, therap +50%). Thirdly, we assessed the function of NK cells exposed in vitro to TOFA and BARI at therap and supra dose with crosslinking NKp30 (CD107a, IFNγ, TNF) and coculture with 2 tumor cell lines (CD107a, cytotoxicity): a lung cancer line A549 and a lymphoma cell line SU-DHL-4
Results: 26 RA patients were included in the ex vivo assay (12 MTX, 10 BARI, 6 TOFA). Patients treated with TOFA had a significant reduced expression of the activation marker CD69 on NK cells compared to MTX (p< 0.05) (Figure 1). After in vitro culture of NK cells with JAKi, we confirmed the negative impact of JAKi on NK cells activation (CD69), maturation (CD57) and activating receptor expression (NKp30), more pronounced with TOFA and UPA with dose-effect (Figure 2). After crosslinking NKp30, NK cells exposed to JAKi expressed less IFNγ, TNF and CD107a compared to DMSO (p< 0.01). When NK cells were cocultured with the tumor cell lines, previous exposition to JAKi led to a significant reduction of CD107a expression on NK cells (p< 0.05) and to reduced cytotoxicity (p< 0.05) with both cell lines at the two concentrations (Figure 3). TOFA was the most impactful JAKi in all experiments. Lastly, we observed a strong correlation between the markers CD69 and NKp30 and functional parameters for each functional assays.
Conclusion: JAKi impairs NK cells phenotype, function and antitumor response both in ex vivo and in vitro experiments with different impact according to the JAKi. The question remains open if this mechanism could explain the increased risk of cancer observed with TOFA in the ORAL surveillance trial. Our data suggest to pursue a careful monitoring of the patients treated with JAKi regarding the risk of cancer.
1Ytterberg et al., N Engl J Med. 2022 ;386(4):316-326.
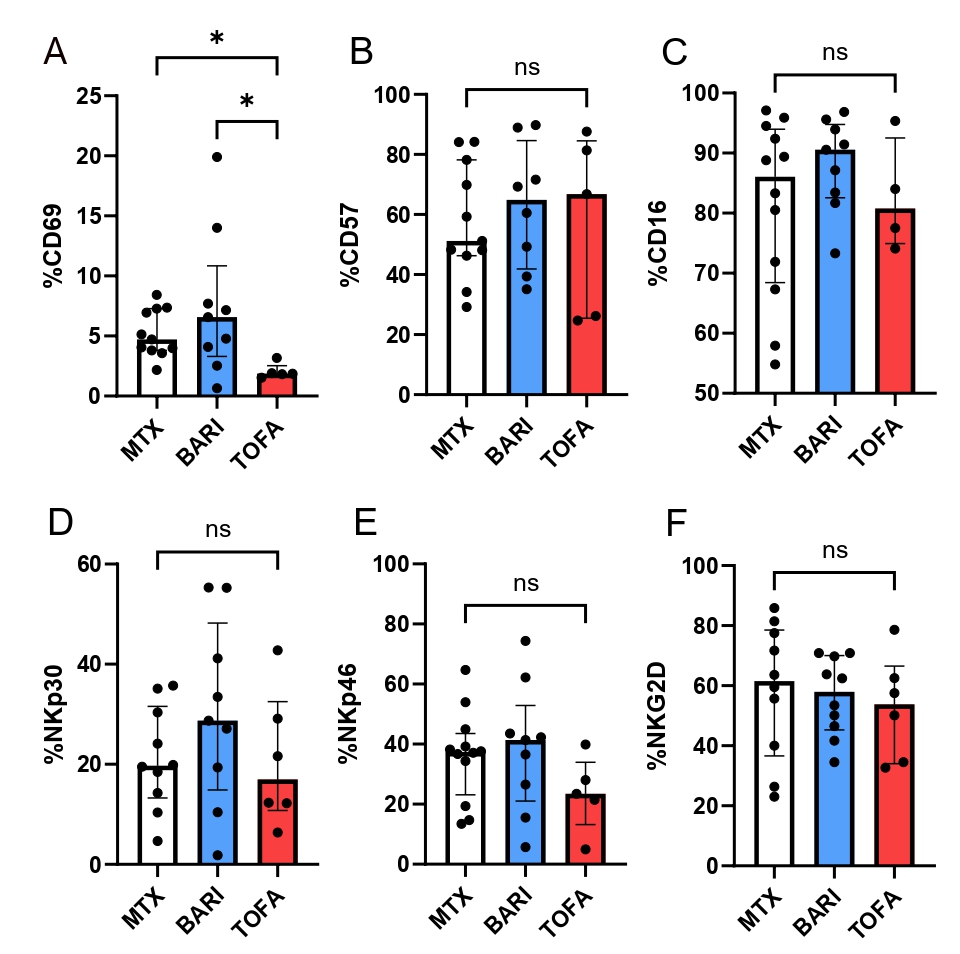 Figure 1: NK cells phenotype in RA patients treated with TOFA, BARI or MTX.
Figure 1: NK cells phenotype in RA patients treated with TOFA, BARI or MTX.
NK cells from RA patients treated by MTX (n=12), TOFA (n=6) and BARI (n=10) were phenotyped. The several parameters have been studied: CD69 (A) CD57 (B) CD16 (C) NKp30 (D) NKp46 (E) NKG2D (F). Kruskal-Wallis test was used. *: p < 0.05.
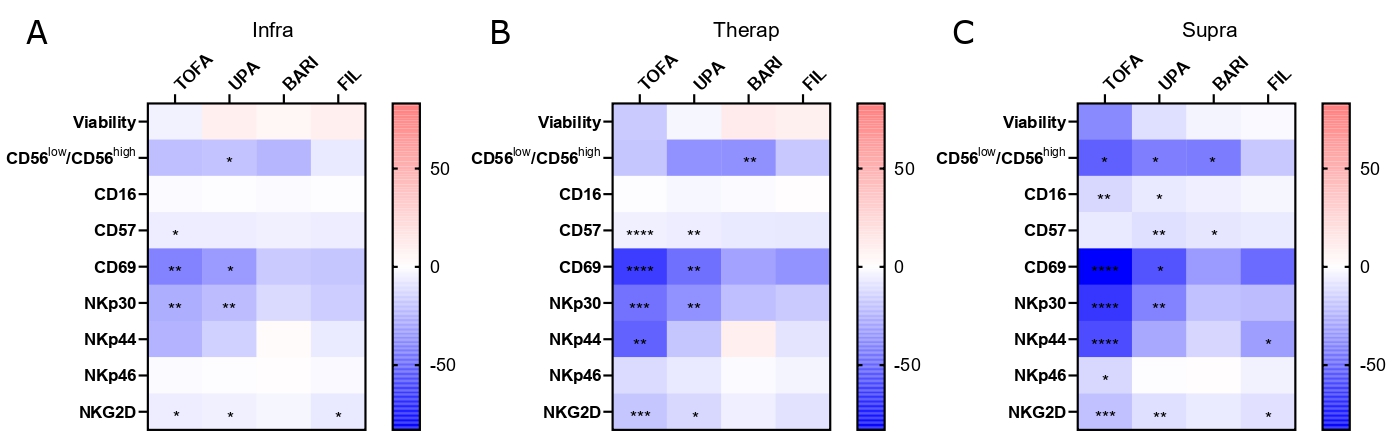 Figure 2: JAKi differently impact NK cells phenotype with a dose effect.
Figure 2: JAKi differently impact NK cells phenotype with a dose effect.
Sorted NK cells from healthy donors were cultured with TOFA, BARI, UPA, FIL at (A) infra (n=7), (B) therap (n=7), (C) supra (n=6) concentrations or DMSO for 6 days and then stained for the following markers: CD16, CD57, CD69, NKp30, NKp44, NKp46 and NKG2D. Results are shown as heatmaps representing the percentage of mean variation compare to DMSO, stars represent the degree of the significance. Friedman test was used. *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001.
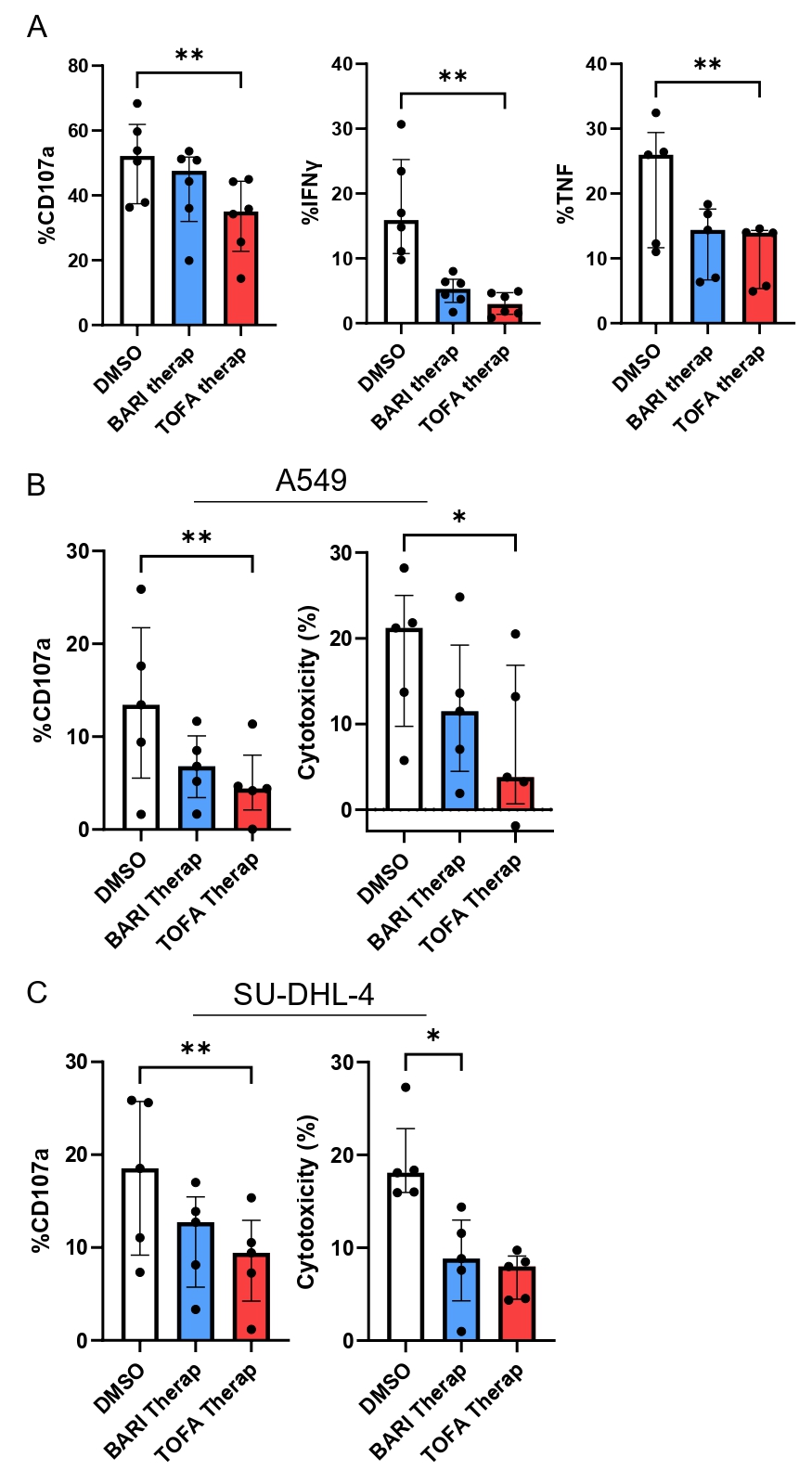 Figure 3: JAKi impairs NK cells function and antitumor activity.
Figure 3: JAKi impairs NK cells function and antitumor activity.
Sorted NK cells from healthy donors were exposed to TOFA or BARI at therap concentrations or DMSO and stimulated by crosslinking with anti-NKp30 (A) to assess CD107a, IFNγ and TNF expression (n=6) and cultured with 2 tumor cell lines, A549 (B) and SU-DHL-4 (C) to assess CD107a expression and cytotoxicity (n=5). Friedman test was used. *: p < 0.05, **: p < 0.01.
Disclosures: L. Meudec, None; P. Richebé, None; J. Pascaud, None; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; G. Nocturne, Pfizer, Novartis, Eli Lilly, Amgen.
Background/Purpose: Janus kinase inhibitors (JAKi) are effective treatments licensed in rheumatoid arthritis (RA). Concerns about a risk of cancer may arise with JAKi as in every immunosuppression situation. Recently the ORAL Surveillance trial comparing tofacitinib (TOFA) to TNF inhibitors (TNFi) in RA patients over fifty years with at least one cardiovascular risk highlighted an increased risk of cancer especially lung cancer and lymphoma1. Given their role in the antitumor surveillance and antiviral response, especially against herpes virus that have been shown to be increased with JAKi, NK cells may play a causal role in these adverse events.
Our hypothesis is that JAKi may promote tumorigenesis by inhibiting NK cells and that this impact could vary depending of the selectivity profile of these drugs. Thus our objective was to assess the phenotypic and functional impact of JAKi on NK cells.
Methods: We first performed ex vivo phenotypic analyses of NK cells in RA patients treated with TOFA or baricitinib (BARI) compared to patients treated with methotrexate (MTX). To go deeper, we exposed in vitro sorted NK cells from healthy donors to the 4 JAKi [(TOFA, BARI, upadacitinib (UPA) and filgotinib (FILGO)] or control (DMSO) for 6 days. We used 3 different doses carefully chosen to mimic in vivo exposure: we first assess the in vitro dose corresponding to the mean dose in patients at licensed dose [therapeutic concentration (therap)], then we framed this dose with a lower dose (infra, therap -50%) and supra dose (supra, therap +50%). Thirdly, we assessed the function of NK cells exposed in vitro to TOFA and BARI at therap and supra dose with crosslinking NKp30 (CD107a, IFNγ, TNF) and coculture with 2 tumor cell lines (CD107a, cytotoxicity): a lung cancer line A549 and a lymphoma cell line SU-DHL-4
Results: 26 RA patients were included in the ex vivo assay (12 MTX, 10 BARI, 6 TOFA). Patients treated with TOFA had a significant reduced expression of the activation marker CD69 on NK cells compared to MTX (p< 0.05) (Figure 1). After in vitro culture of NK cells with JAKi, we confirmed the negative impact of JAKi on NK cells activation (CD69), maturation (CD57) and activating receptor expression (NKp30), more pronounced with TOFA and UPA with dose-effect (Figure 2). After crosslinking NKp30, NK cells exposed to JAKi expressed less IFNγ, TNF and CD107a compared to DMSO (p< 0.01). When NK cells were cocultured with the tumor cell lines, previous exposition to JAKi led to a significant reduction of CD107a expression on NK cells (p< 0.05) and to reduced cytotoxicity (p< 0.05) with both cell lines at the two concentrations (Figure 3). TOFA was the most impactful JAKi in all experiments. Lastly, we observed a strong correlation between the markers CD69 and NKp30 and functional parameters for each functional assays.
Conclusion: JAKi impairs NK cells phenotype, function and antitumor response both in ex vivo and in vitro experiments with different impact according to the JAKi. The question remains open if this mechanism could explain the increased risk of cancer observed with TOFA in the ORAL surveillance trial. Our data suggest to pursue a careful monitoring of the patients treated with JAKi regarding the risk of cancer.
1Ytterberg et al., N Engl J Med. 2022 ;386(4):316-326.
 Figure 1: NK cells phenotype in RA patients treated with TOFA, BARI or MTX.
Figure 1: NK cells phenotype in RA patients treated with TOFA, BARI or MTX.NK cells from RA patients treated by MTX (n=12), TOFA (n=6) and BARI (n=10) were phenotyped. The several parameters have been studied: CD69 (A) CD57 (B) CD16 (C) NKp30 (D) NKp46 (E) NKG2D (F). Kruskal-Wallis test was used. *: p < 0.05.
 Figure 2: JAKi differently impact NK cells phenotype with a dose effect.
Figure 2: JAKi differently impact NK cells phenotype with a dose effect.Sorted NK cells from healthy donors were cultured with TOFA, BARI, UPA, FIL at (A) infra (n=7), (B) therap (n=7), (C) supra (n=6) concentrations or DMSO for 6 days and then stained for the following markers: CD16, CD57, CD69, NKp30, NKp44, NKp46 and NKG2D. Results are shown as heatmaps representing the percentage of mean variation compare to DMSO, stars represent the degree of the significance. Friedman test was used. *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001.
 Figure 3: JAKi impairs NK cells function and antitumor activity.
Figure 3: JAKi impairs NK cells function and antitumor activity.Sorted NK cells from healthy donors were exposed to TOFA or BARI at therap concentrations or DMSO and stimulated by crosslinking with anti-NKp30 (A) to assess CD107a, IFNγ and TNF expression (n=6) and cultured with 2 tumor cell lines, A549 (B) and SU-DHL-4 (C) to assess CD107a expression and cytotoxicity (n=5). Friedman test was used. *: p < 0.05, **: p < 0.01.
Disclosures: L. Meudec, None; P. Richebé, None; J. Pascaud, None; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; G. Nocturne, Pfizer, Novartis, Eli Lilly, Amgen.

