Back
Poster Session B
Juvenile idiopathic arthritis (JIA) and pediatric joint disorders
Session: (0850–0880) Pediatric Rheumatology – Clinical Poster I: JIA
0879: Golimumab for Treatment of Polyarticular Juvenile Idiopathic Arthritis - Safety and Effectiveness Updates from a Comparative Study
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- AZ
Angela Zimmer, MD
Asklepios
St. Augustin, Germany
Abstract Poster Presenter(s)
Angela Zimmer1, Ariane Klein1 and Gerd Horneff2, 1Asklepios, St. Augustin, Germany, 2Pediatrics, Asklepios Klinik Sankt Augustin GmbH, Sankt Augustin, Germany
Background/Purpose: Golimumab (GOL) is approved for the treatment of polyarticular juvenile idiopathic arthritis (pJIA) in patients 2 years and older. Data on long-term safety and efficacy of GOL in this indication are limited.
Methods: In this ongoing non-interventional observational study, clinical characteristics, disease activity and safety parameters were analyzed using the German Biologics in Paediatric Rheumatology (BiKeR) registry. One hundred twenty-four pJIA-patients treated with GOL were body weight-matched with 248 patients receiving alternative tumor necrosis factor inhibitors (aTNFi) and 174 biologic–naïve patients under methotrexate (MTX) therapy.
Results: Baseline parameters of the GOL cohort differed from the aTNFi and MTX groups with longer disease duration, lower age at disease onset and higher number of previously failing disease modifying antirheumatic drugs (DMARDs). Compared to the MTX cohort, GOL patients more often displayed extended oligoarthritis (eoJIA) and less frequently rheumatoid factor negative polyarthritis (RF-PA). GOL patients were diagnosed with a rheumatoid factor positive polyarthritis less often than patients in the aTNFi cohort. Patients in the GOL and aTNFi cohorts had comparable baseline disease activity characteristics, which were notably lower than the MTX cohort (table 1). The long-term clinical effectiveness of GOL in pJIA was shown by a decrease of the mean JADAS 10 from 11.3 ± 6.6 at baseline to 4.2 ± 5.3 after 24 months. After 2 years, a JADAS 10 minimal disease activity was reached by 54.8 % of patients, whereas 38.7 % of patients were in remission and the JIA ACR 30/50/70/90 response rates were71.0/67.7/61.3/48.4% respectively (table 2). Adverse event incidence rates and especially serious infection (SI) rates were comparable between the three cohorts. In the GOL cohort 12 SAE were reported, while in the aTNFi cohort 21 SAEs and in the MTX cohort 9 SAE were noted. One SI event was documented in the GOL cohort, 5 in the aTNFi cohort and 2 in the MTX cohort, none were unexpected (table 3). Few autoimmune processes occurred: 4 incident events in the GOL cohort, 9 cases in the aTNFi cohort and 11 events in MTX cohort (table 3). Of the patients with a preexisting uveitis at baseline, 8 of 37 in the GOL cohort and 4 of 28 of the aTNFi cohort had uveitis flares. New occurrences of uveitis were infrequent, with a total of 16 incident cases across all cohorts (1 in GOL, 5 in aTNFi and 10 in MTX). No malignancies or deaths were reported.
Conclusion: Our interim results show an acceptable safety profile of GOL therapy, comparable to treatment with aTNFi and MTX. The outcome data confirm long-term benefits of GOL treatment in pJIA patients.
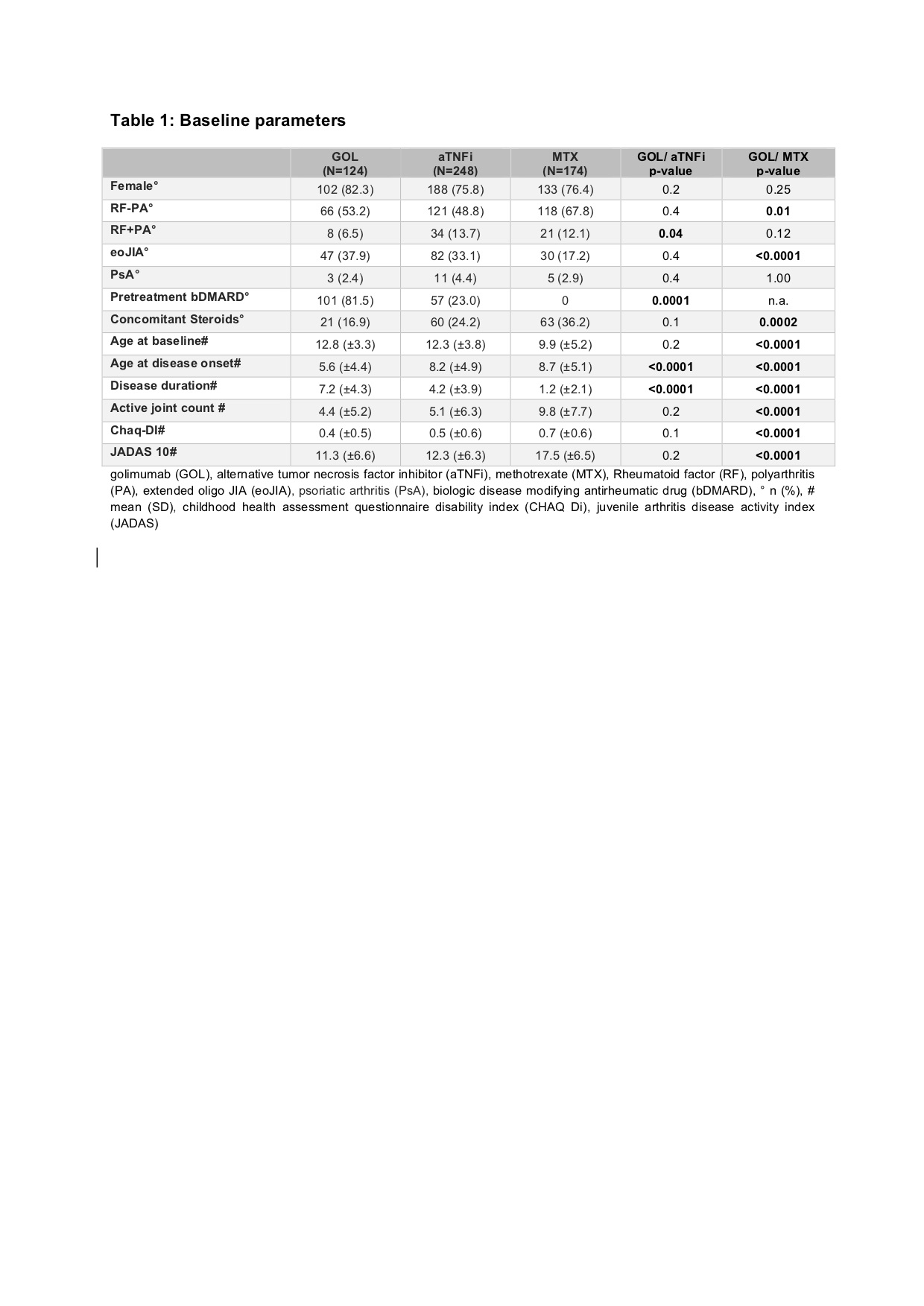 golimumab (GOL), alternative tumor necrosis factor inhibitor (aTNFi), methotrexate (MTX), Rheumatoid factor (RF), polyarthritis (PA), extended oligo JIA (eoJIA), psoriatic arthritis (PsA), biologic disease modifying antirheumatic drug (bDMARD), ° n (%), # mean (SD), childhood health assessment questionnaire disability index (CHAQ Di), juvenile arthritis disease activity index (JADAS)
golimumab (GOL), alternative tumor necrosis factor inhibitor (aTNFi), methotrexate (MTX), Rheumatoid factor (RF), polyarthritis (PA), extended oligo JIA (eoJIA), psoriatic arthritis (PsA), biologic disease modifying antirheumatic drug (bDMARD), ° n (%), # mean (SD), childhood health assessment questionnaire disability index (CHAQ Di), juvenile arthritis disease activity index (JADAS)
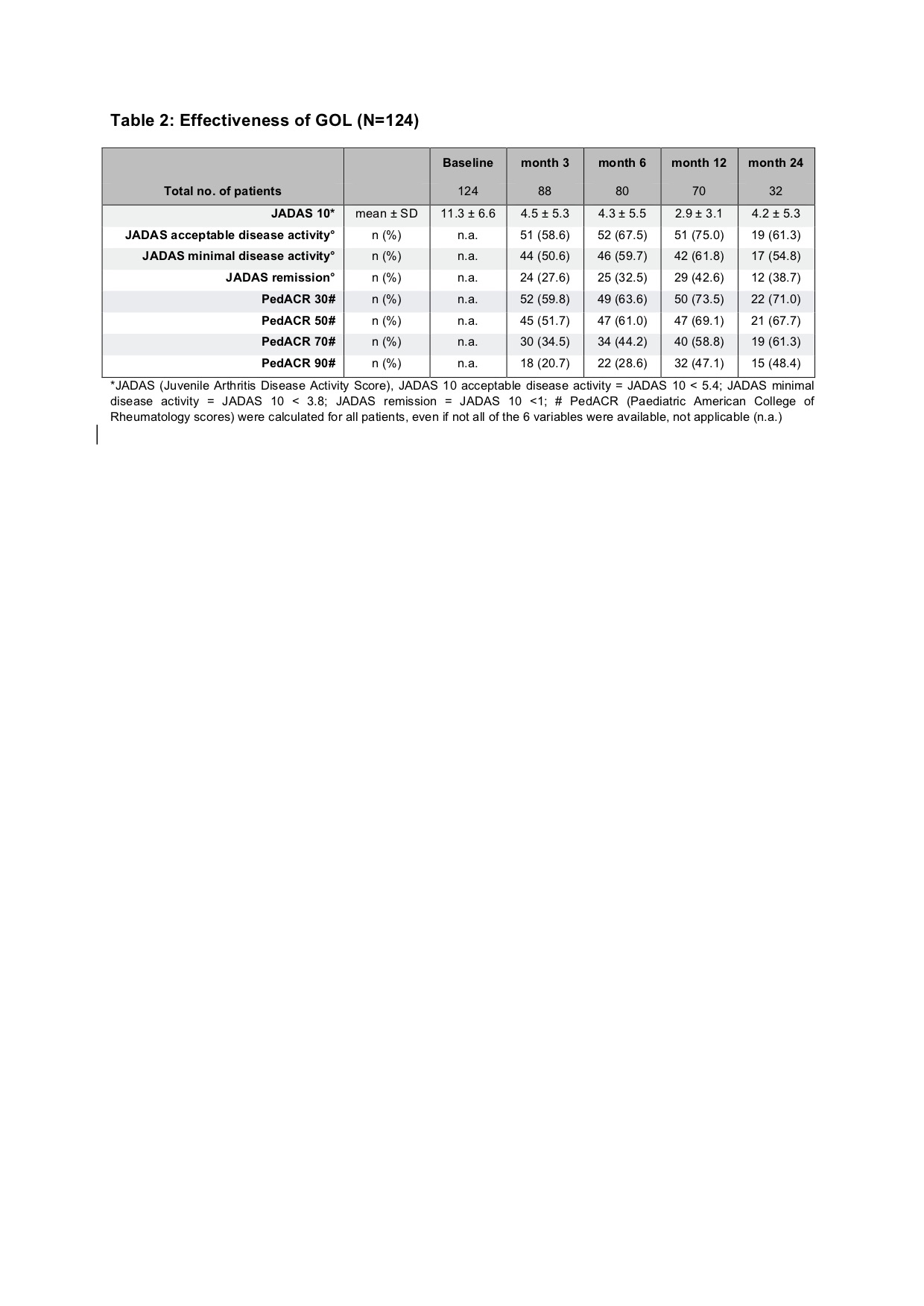 *JADAS (Juvenile Arthritis Disease Activity Score), JADAS 10 acceptable disease activity = JADAS 10 < 5.4; JADAS minimal disease activity = JADAS 10 < 3.8; JADAS remission = JADAS 10 < 1; # PedACR (Paediatric American College of Rheumatology scores) were calculated for all patients, even if not all of the 6 variables were available, not applicable (n.a.)
*JADAS (Juvenile Arthritis Disease Activity Score), JADAS 10 acceptable disease activity = JADAS 10 < 5.4; JADAS minimal disease activity = JADAS 10 < 3.8; JADAS remission = JADAS 10 < 1; # PedACR (Paediatric American College of Rheumatology scores) were calculated for all patients, even if not all of the 6 variables were available, not applicable (n.a.)
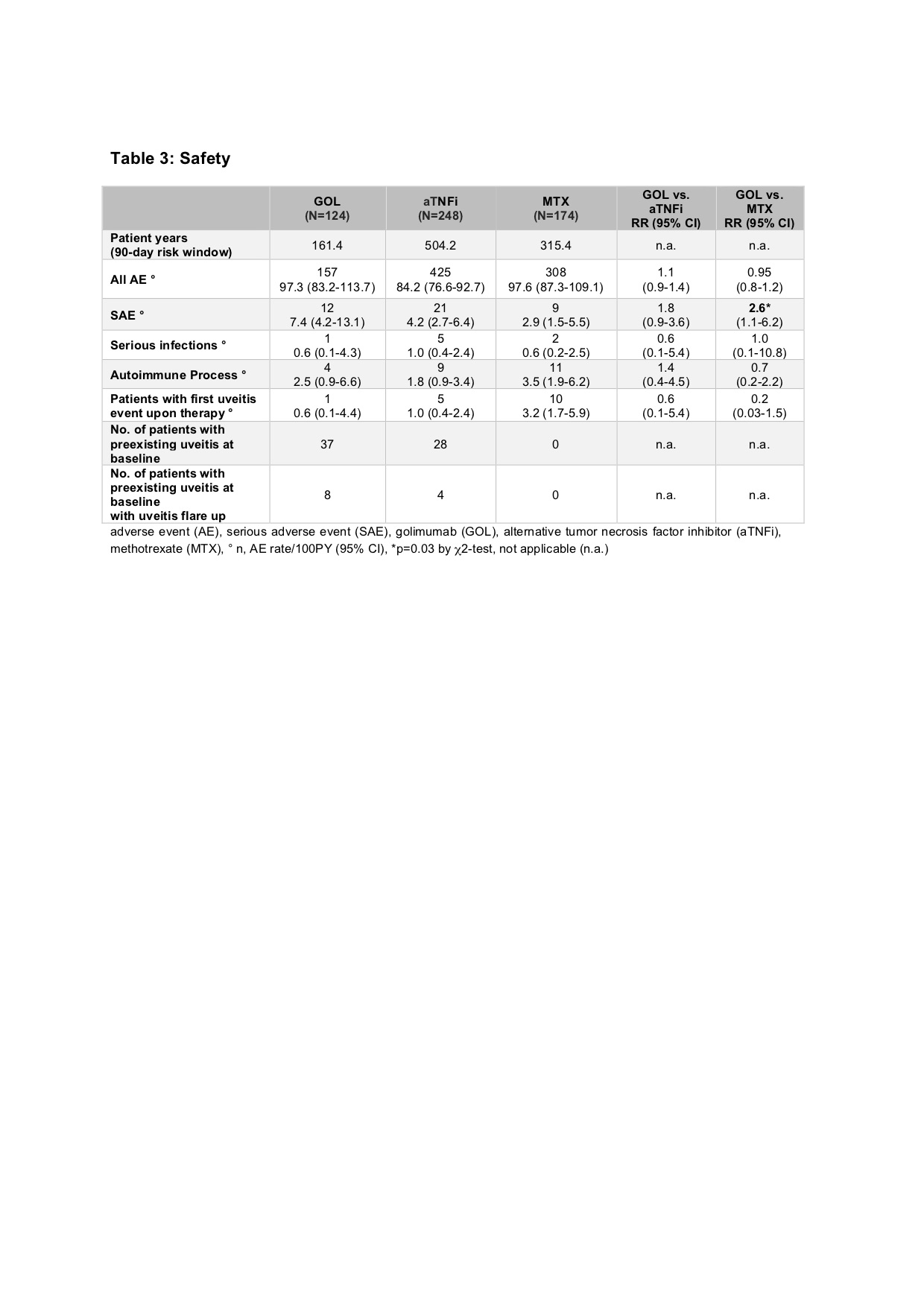 adverse event (AE), serious adverse event (SAE), golimumab (GOL), alternative tumor necrosis factor inhibitor (aTNFi), methotrexate (MTX), ° n, AE rate/100PY (95% CI), *p=0.03 by χ2-test, not applicable (n.a.)
adverse event (AE), serious adverse event (SAE), golimumab (GOL), alternative tumor necrosis factor inhibitor (aTNFi), methotrexate (MTX), ° n, AE rate/100PY (95% CI), *p=0.03 by χ2-test, not applicable (n.a.)
Disclosures: A. Zimmer, None; A. Klein, None; G. Horneff, Roche, Pfizer, Novartis, Merck/MSD, Eli Lilly, AbbVie/Abbott.
Background/Purpose: Golimumab (GOL) is approved for the treatment of polyarticular juvenile idiopathic arthritis (pJIA) in patients 2 years and older. Data on long-term safety and efficacy of GOL in this indication are limited.
Methods: In this ongoing non-interventional observational study, clinical characteristics, disease activity and safety parameters were analyzed using the German Biologics in Paediatric Rheumatology (BiKeR) registry. One hundred twenty-four pJIA-patients treated with GOL were body weight-matched with 248 patients receiving alternative tumor necrosis factor inhibitors (aTNFi) and 174 biologic–naïve patients under methotrexate (MTX) therapy.
Results: Baseline parameters of the GOL cohort differed from the aTNFi and MTX groups with longer disease duration, lower age at disease onset and higher number of previously failing disease modifying antirheumatic drugs (DMARDs). Compared to the MTX cohort, GOL patients more often displayed extended oligoarthritis (eoJIA) and less frequently rheumatoid factor negative polyarthritis (RF-PA). GOL patients were diagnosed with a rheumatoid factor positive polyarthritis less often than patients in the aTNFi cohort. Patients in the GOL and aTNFi cohorts had comparable baseline disease activity characteristics, which were notably lower than the MTX cohort (table 1). The long-term clinical effectiveness of GOL in pJIA was shown by a decrease of the mean JADAS 10 from 11.3 ± 6.6 at baseline to 4.2 ± 5.3 after 24 months. After 2 years, a JADAS 10 minimal disease activity was reached by 54.8 % of patients, whereas 38.7 % of patients were in remission and the JIA ACR 30/50/70/90 response rates were71.0/67.7/61.3/48.4% respectively (table 2). Adverse event incidence rates and especially serious infection (SI) rates were comparable between the three cohorts. In the GOL cohort 12 SAE were reported, while in the aTNFi cohort 21 SAEs and in the MTX cohort 9 SAE were noted. One SI event was documented in the GOL cohort, 5 in the aTNFi cohort and 2 in the MTX cohort, none were unexpected (table 3). Few autoimmune processes occurred: 4 incident events in the GOL cohort, 9 cases in the aTNFi cohort and 11 events in MTX cohort (table 3). Of the patients with a preexisting uveitis at baseline, 8 of 37 in the GOL cohort and 4 of 28 of the aTNFi cohort had uveitis flares. New occurrences of uveitis were infrequent, with a total of 16 incident cases across all cohorts (1 in GOL, 5 in aTNFi and 10 in MTX). No malignancies or deaths were reported.
Conclusion: Our interim results show an acceptable safety profile of GOL therapy, comparable to treatment with aTNFi and MTX. The outcome data confirm long-term benefits of GOL treatment in pJIA patients.
 golimumab (GOL), alternative tumor necrosis factor inhibitor (aTNFi), methotrexate (MTX), Rheumatoid factor (RF), polyarthritis (PA), extended oligo JIA (eoJIA), psoriatic arthritis (PsA), biologic disease modifying antirheumatic drug (bDMARD), ° n (%), # mean (SD), childhood health assessment questionnaire disability index (CHAQ Di), juvenile arthritis disease activity index (JADAS)
golimumab (GOL), alternative tumor necrosis factor inhibitor (aTNFi), methotrexate (MTX), Rheumatoid factor (RF), polyarthritis (PA), extended oligo JIA (eoJIA), psoriatic arthritis (PsA), biologic disease modifying antirheumatic drug (bDMARD), ° n (%), # mean (SD), childhood health assessment questionnaire disability index (CHAQ Di), juvenile arthritis disease activity index (JADAS)  *JADAS (Juvenile Arthritis Disease Activity Score), JADAS 10 acceptable disease activity = JADAS 10 < 5.4; JADAS minimal disease activity = JADAS 10 < 3.8; JADAS remission = JADAS 10 < 1; # PedACR (Paediatric American College of Rheumatology scores) were calculated for all patients, even if not all of the 6 variables were available, not applicable (n.a.)
*JADAS (Juvenile Arthritis Disease Activity Score), JADAS 10 acceptable disease activity = JADAS 10 < 5.4; JADAS minimal disease activity = JADAS 10 < 3.8; JADAS remission = JADAS 10 < 1; # PedACR (Paediatric American College of Rheumatology scores) were calculated for all patients, even if not all of the 6 variables were available, not applicable (n.a.) adverse event (AE), serious adverse event (SAE), golimumab (GOL), alternative tumor necrosis factor inhibitor (aTNFi), methotrexate (MTX), ° n, AE rate/100PY (95% CI), *p=0.03 by χ2-test, not applicable (n.a.)
adverse event (AE), serious adverse event (SAE), golimumab (GOL), alternative tumor necrosis factor inhibitor (aTNFi), methotrexate (MTX), ° n, AE rate/100PY (95% CI), *p=0.03 by χ2-test, not applicable (n.a.) Disclosures: A. Zimmer, None; A. Klein, None; G. Horneff, Roche, Pfizer, Novartis, Merck/MSD, Eli Lilly, AbbVie/Abbott.

