Back
Poster Session D
Session: (1830–1855) Miscellaneous Rheumatic and Inflammatory Diseases Poster III
1850: Guselkumab Provides Sustained Improvements in Health-Related Quality of Life in Patients with Active Psoriatic Arthritis Through 2 Years of DISCOVER-2
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Jeffrey Curtis, MD, MS, MPH
University of Alabama at Birmingham
Hoover, AL, United States
Abstract Poster Presenter(s)
Jeffrey Curtis1, Iain B McInnes2, Proton Rahman3, Dafna Gladman4, Feifei Yang5, Steve Peterson5, Alexa Kollmeier6, Natalie Shiff7, Chenglong Han8, May Shawi9, William Tillett10 and Philip J Mease11, 1Division of Clinical Immunology & Rheumatology, University of Alabama at Birmingham, Birmingham, AL, 2Institute of Infection, Immunity and Inflammation, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, Scotland, United Kingdom, 3Memorial University, St. John's, NL, Canada, 4Toronto Western Hospital, Schroeder Arthritis Institute, Toronto, ON, Canada, 5Department of Immunology, Janssen Global Services, LLC, Horsham, PA, 6Janssen-Cilag, Research & Development, LLC, San Diego, CA, 7Janssen Scientific Affairs, LLC; Community Health and Epidemiology, University of Saskatchewan, Philadelphia, PA, 8Immunology, Janssen Research & Development, LLC, Spring House, PA, 9Immunology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson, Horsham, PA, 10Royal National Hospital for Rheumatic Diseases, Bath, United Kingdom, 11Swedish Medical Center/Providence St. Joseph Health, Seattle, WA
Background/Purpose: Psoriatic arthritis (PsA), a chronic inflammatory disease characterized by peripheral arthritis, axial inflammation, dactylitis, enthesitis, and skin/nail psoriasis, is associated with reduced health-related quality of life (HRQoL). This study assessed long-term effect of guselkumab (GUS), a human monoclonal antibody that selectively targets the interleukin (IL)-23p19 subunit, on HRQoL of bio-naïve PsA patients who participated in the phase 3 DISCOVER-2 trial.1
Methods: Patients with active PsA despite nonbiologic disease-modifying antirheumatic drugs (DMARDs) and/or nonsteroidal anti-inflammatory drugs (NSAIDs) received GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at Week (W) 0, W4, then Q8W; or placebo (PBO). At W24, PBO patients crossed over to GUS 100 mg Q4W. HRQoL was assessed using the patient-reported EuroQoL-5 Dimension-5 Level (EQ-5D-5L) questionnaire index and EuroQol Visual Analog Scale (EQ-VAS), widely used and complimentary tools that allow patients to provide a global assessment of their HRQoL. The EQ-5D-5L index assesses mobility, self-care, usual activities, pain/discomfort, and anxiety/depression; an index score is derived ranging from 0 (death) to 1 (perfect health).2 EQ‑VAS assesses patient health state on a scale of 0-100, with higher scores indicating better health. Using mixed effects models for repeated measures (MMRM), least squares (LS) mean changes from baseline in the EQ-5D-5L index and EQ‑VAS through W100 were assessed. Observed changes from baseline were evaluated; in patients who met treatment failure rules before W24 and in patients who discontinued with missing data after W24, changes from baseline were imputed as 0.
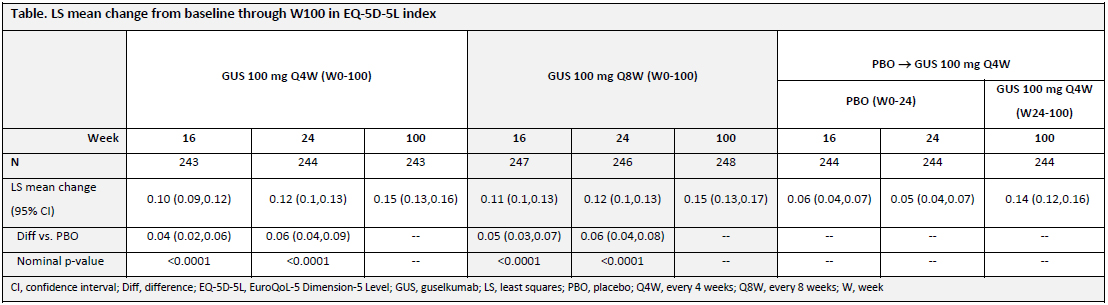
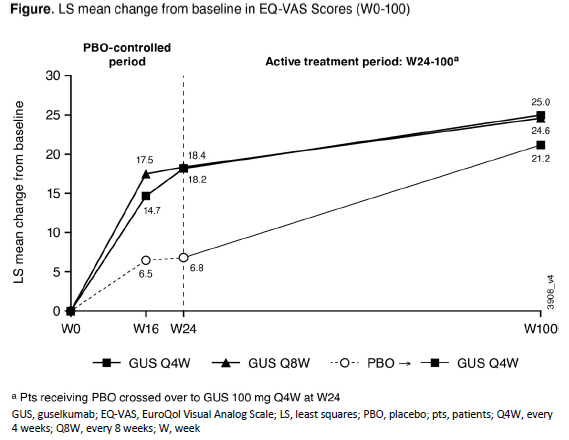
Results: GUS-treated patients achieved greater improvements in patient-reported health status than PBO at both W16 and W24 when evaluated using both the EQ-5D-5L index score and the EQ-VAS. The improvements by GUS in EQ-5D-5L index scores through W24 (0.12 for GUS Q4W/Q8W vs 0.05 for PBO; each nominal p< 0.0001) were maintained with continued GUS through 2 years (0.15 for GUS Q4W/Q8W) (Table). PBO-treated patients who started GUS at W24 reported comparable improvements in their HRQoL by W52 (0.12), with maintenance though W100 (0.14). Similar results were observed with EQ-VAS (Figure). W24 improvements in EQ-VAS scores were greater following GUS treatment (18.2/18.4 GUS Q4W/Q8W) vs PBO (6.8; nominal p< 0.0001). EQ-VAS scores continued to improve with GUS through 2 years (25.0/24.6 GUS Q4W/Q8W). Likewise, PBO-treated patients who crossed over to GUS at W24 experienced improvements in HRQoL by W52 (18.8), with maintenance through W100 (21.2).
Conclusion: In bio-naïve patients with active PsA receiving GUS, earlier improvements (at the first timepoint assessed) in self-reported health-related measures were sustained through 2 years.
References:
1. Mease PJ, et al. Lancet. 2020;395:1126–36.
2. EuroQol Group. Health Policy. 1990;16:199-208.
Disclosures: J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; I. McInnes, Bristol-Myers Squibb (BMS), Janssen, Novartis, UCB, Pfizer, AbbVie, Celgene, AstraZeneca, Boehringer Ingelheim, EveloBio, LEO, Lilly; P. Rahman, Janssen, Novartis, AbbVie, Eli Lilly and Company, Pfizer; D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; F. Yang, Janssen Global Services, LLC; S. Peterson, Johnson & Johnson; A. Kollmeier, Janssen Research & Development, LLC, Johnson & Johnson; N. Shiff, AbbVie/Abbott, Gilead, Johnson & Johnson, Janssen Scientific Affairs, LLC; C. Han, Janssen Research and Development, LLC, Johnson & Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; W. Tillett, AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.
Background/Purpose: Psoriatic arthritis (PsA), a chronic inflammatory disease characterized by peripheral arthritis, axial inflammation, dactylitis, enthesitis, and skin/nail psoriasis, is associated with reduced health-related quality of life (HRQoL). This study assessed long-term effect of guselkumab (GUS), a human monoclonal antibody that selectively targets the interleukin (IL)-23p19 subunit, on HRQoL of bio-naïve PsA patients who participated in the phase 3 DISCOVER-2 trial.1
Methods: Patients with active PsA despite nonbiologic disease-modifying antirheumatic drugs (DMARDs) and/or nonsteroidal anti-inflammatory drugs (NSAIDs) received GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at Week (W) 0, W4, then Q8W; or placebo (PBO). At W24, PBO patients crossed over to GUS 100 mg Q4W. HRQoL was assessed using the patient-reported EuroQoL-5 Dimension-5 Level (EQ-5D-5L) questionnaire index and EuroQol Visual Analog Scale (EQ-VAS), widely used and complimentary tools that allow patients to provide a global assessment of their HRQoL. The EQ-5D-5L index assesses mobility, self-care, usual activities, pain/discomfort, and anxiety/depression; an index score is derived ranging from 0 (death) to 1 (perfect health).2 EQ‑VAS assesses patient health state on a scale of 0-100, with higher scores indicating better health. Using mixed effects models for repeated measures (MMRM), least squares (LS) mean changes from baseline in the EQ-5D-5L index and EQ‑VAS through W100 were assessed. Observed changes from baseline were evaluated; in patients who met treatment failure rules before W24 and in patients who discontinued with missing data after W24, changes from baseline were imputed as 0.
Results: GUS-treated patients achieved greater improvements in patient-reported health status than PBO at both W16 and W24 when evaluated using both the EQ-5D-5L index score and the EQ-VAS. The improvements by GUS in EQ-5D-5L index scores through W24 (0.12 for GUS Q4W/Q8W vs 0.05 for PBO; each nominal p< 0.0001) were maintained with continued GUS through 2 years (0.15 for GUS Q4W/Q8W) (Table). PBO-treated patients who started GUS at W24 reported comparable improvements in their HRQoL by W52 (0.12), with maintenance though W100 (0.14). Similar results were observed with EQ-VAS (Figure). W24 improvements in EQ-VAS scores were greater following GUS treatment (18.2/18.4 GUS Q4W/Q8W) vs PBO (6.8; nominal p< 0.0001). EQ-VAS scores continued to improve with GUS through 2 years (25.0/24.6 GUS Q4W/Q8W). Likewise, PBO-treated patients who crossed over to GUS at W24 experienced improvements in HRQoL by W52 (18.8), with maintenance through W100 (21.2).
Conclusion: In bio-naïve patients with active PsA receiving GUS, earlier improvements (at the first timepoint assessed) in self-reported health-related measures were sustained through 2 years.
References:
1. Mease PJ, et al. Lancet. 2020;395:1126–36.
2. EuroQol Group. Health Policy. 1990;16:199-208.


Disclosures: J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; I. McInnes, Bristol-Myers Squibb (BMS), Janssen, Novartis, UCB, Pfizer, AbbVie, Celgene, AstraZeneca, Boehringer Ingelheim, EveloBio, LEO, Lilly; P. Rahman, Janssen, Novartis, AbbVie, Eli Lilly and Company, Pfizer; D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; F. Yang, Janssen Global Services, LLC; S. Peterson, Johnson & Johnson; A. Kollmeier, Janssen Research & Development, LLC, Johnson & Johnson; N. Shiff, AbbVie/Abbott, Gilead, Johnson & Johnson, Janssen Scientific Affairs, LLC; C. Han, Janssen Research and Development, LLC, Johnson & Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; W. Tillett, AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.

