Back
Ignite Talk
Session: Ignite Session 8B
0144: The Association of Hypocomplementemia with Organ Involvement and Serum IgG4 in IgG4-related Disease
Monday, November 14, 2022
2:05 PM – 2:10 PM Eastern Time
Location: Center City Stage
- GK
Guy Katz, MD
Massachusetts General Hospital
Boston, MA, United StatesDisclosure: Disclosure(s): No financial relationships with ineligible companies to disclose
Ignite Speaker(s)
Guy Katz1, Amelia S. Cogan1, Grace McMahon1, Sebastian Perez-Espina1, Ana Fernandes1, Cory Perugino1, Zachary Wallace1, John Atkinson2, Alfred Kim2 and John Stone3, 1Massachusetts General Hospital, Boston, MA, 2Washington University School of Medicine, St. Louis, MO, 3Massachusetts General Hospital Rheumatology Unit, Harvard Medical School, Boston, MA
Background/Purpose: IgG4-related disease (IgG4-RD) is a systemic immune-mediated disease that can affect nearly every organ in the body. Hypocomplementemia is common in some patients with IgG4-RD, suggesting that complement activation may be involved in the disease pathophysiology. Hypocomplementemia may be especially common in patients with renal involvement of IgG4-RD. We aimed to evaluate the association of patient characteristics with hypocomplementemia in our large IgG4-RD cohort.
Methods: Patients identified through the Massachusetts General Hospital IgG4-RD cohort were included in this study if they fulfilled the 2019 ACR/EULAR IgG4-RD Classification Criteria or had “probable” IgG4-RD. Probable IgG4-RD is defined as: 1) typical organ involvement; 2) no exclusion criteria present; 3) insufficient total classification points (< 20). The usual scenarios for a diagnosis of probable IgG4-RD are when biopsy is non-diagnostic or not obtained. Hypocomplementemia was defined as any measurement of serum C3 or C4 complement below the lower limit of normal (LLN). Disease characteristics were extracted via review of medical records. Statistical analyses were unpaired T-tests and Chi-squared tests.
Results: Hypocomplementemia was identified in 91 (30%) of 306 patients in the cohort. Characteristics of patients with and without hypocomplementemia are summarized in Table 1. Patients with hypocomplementemia were less likely to have normal serum IgG4 (11% vs. 26%, p=0.009) and more likely to have highly elevated (above 5x the upper limit of normal) serum IgG4 (52% vs. 28%, p=0.001). Patients with hypocomplementemia had more organs involved (mean 4.3 vs. 2.9, p< 0.001). Among patients with hypocomplementemia, C3 was low in 79%, C4 in 84%, and both in 64%. 17% of C3 troughs and 43% of C4 troughs were at or below one-half of the LLN for the assay.
Patients with vs. without hypocomplementemia more commonly had submandibular gland, lymph node, lung, liver, and renal involvement as well as constitutional symptoms (Figure 1). Retroperitoneal involvement was unusual among hypocomplementemic patients. Of the 84 patients with IgG4-related kidney disease (IgG4-RKD), 38 (45%) had hypocomplementemia. Hypocomplementemic patients with IgG4-RKD had significantly more organs involved (mean 5.2 vs. 3.7, p=0.005), more commonly had both low C3 and C4 complement (84% vs. 47%, p=0.03), and more commonly had C3 or C4 troughs at or below one-half of the LLN (24% vs. 6%, p=0.02 for C3; 66% vs. 26%, p=0.003 for C4) than those without IgG4-RKD (Table 2).
Conclusion: Hypocomplementemia is common, and often profound, in IgG4-RD and is associated with serum IgG4 concentration and multiorgan involvement. Additional investigations are needed to describe the nature of complement activation and its role in disease activity, both in the kidneys and beyond.
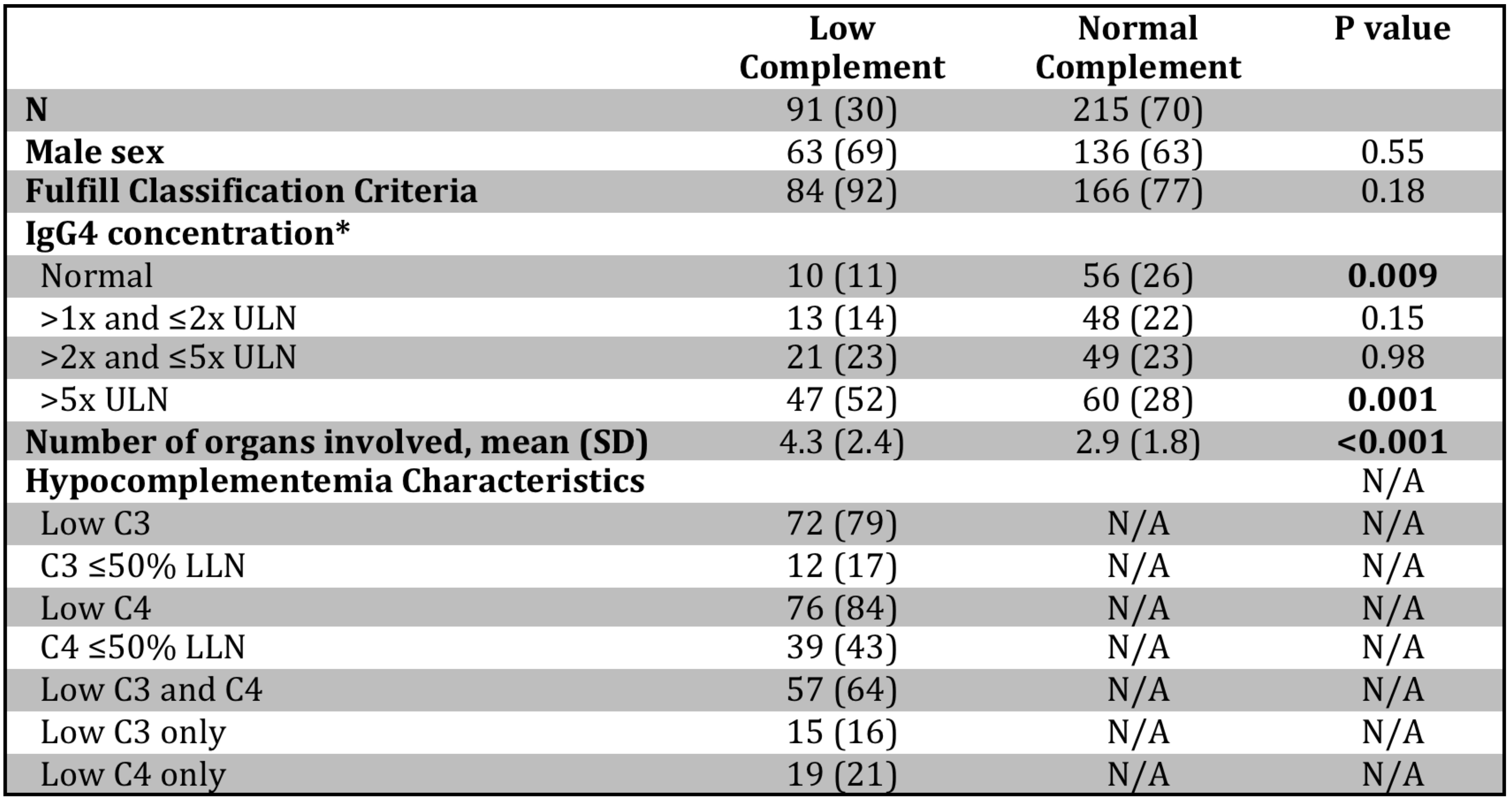 Table 1. Characteristics of IgG4-RD patients with and without hypocomplementemia. *IgG4 concentration was not available for one patient without hypocomplementemia. Values are reported as n (%) unless noted otherwise. ULN: upper limit of normal, LLN: lower limit of normal; SD: standard deviation.
Table 1. Characteristics of IgG4-RD patients with and without hypocomplementemia. *IgG4 concentration was not available for one patient without hypocomplementemia. Values are reported as n (%) unless noted otherwise. ULN: upper limit of normal, LLN: lower limit of normal; SD: standard deviation.
.jpg) Figure 1. IgG4-RD manifestations in patients with and without hypocomplementemia. *Signifies P < 0.05.
Figure 1. IgG4-RD manifestations in patients with and without hypocomplementemia. *Signifies P < 0.05.
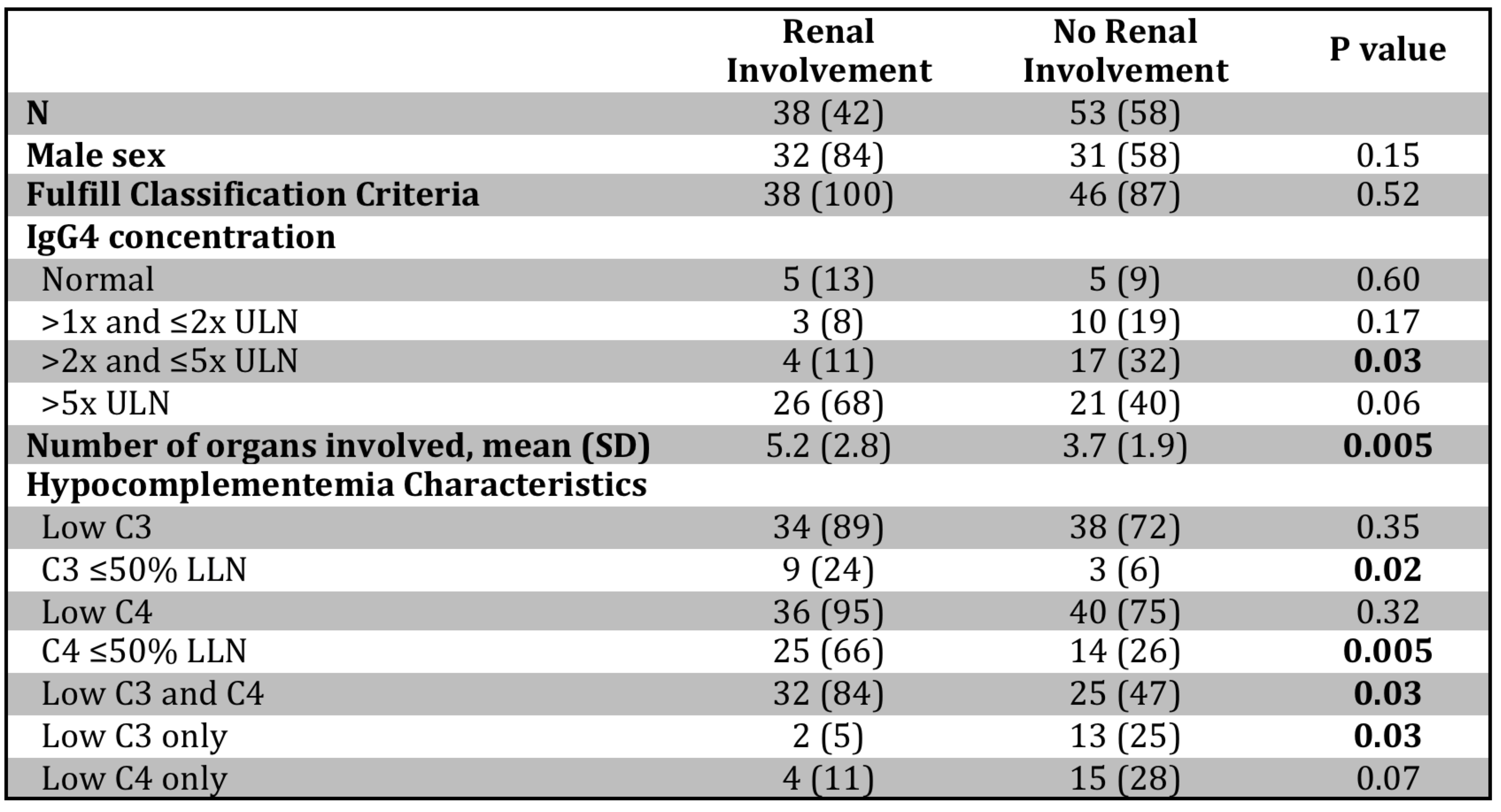 Table 2. Hypocomplementemic patients with and without IgG4-related kidney disease. Values are reported as n (%) unless noted otherwise. ULN: upper limit of normal, LLN: lower limit of normal; SD: standard deviation.
Table 2. Hypocomplementemic patients with and without IgG4-related kidney disease. Values are reported as n (%) unless noted otherwise. ULN: upper limit of normal, LLN: lower limit of normal; SD: standard deviation.
Disclosures: G. Katz, None; A. Cogan, None; G. McMahon, None; S. Perez-Espina, None; A. Fernandes, None; C. Perugino, None; Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Horizon, Shionogi; J. Atkinson, Compliment Corporation, Kypha, Inc., Gemini Therapeutics, Q32 Bio, Celldex Therapeutics, Clinical Pharmacy Services, CDMI, Kairos Bioconsulting, LLC, Achillion Pharmaceuticals, Inc, BioMarin Pharmaceutical Inc., Annexon Biosciences, Inc., Alexion Pharmaceuticals, Broadwing Bio, LLC; A. Kim, GlaxoSmithKline, Kypha Inc., Foghorn Therapeutics, Aurinia Pharmaceuticals, Exagen Diagnostics, Alexion Pharmaceuticals, Pfizer, AstraZeneca; J. Stone, Horizon Theraputics, Sanofi, Amgen, Argenx, Bristol-Myers Squibb(BMS), Chemocentryx, Kyverna, Novartis, Palleon Pharmaceuticals, PPD, Q32, Star Therapeutics, Roche, Mirabio, Spruce Biosciences, Steritas, Zenas.
Background/Purpose: IgG4-related disease (IgG4-RD) is a systemic immune-mediated disease that can affect nearly every organ in the body. Hypocomplementemia is common in some patients with IgG4-RD, suggesting that complement activation may be involved in the disease pathophysiology. Hypocomplementemia may be especially common in patients with renal involvement of IgG4-RD. We aimed to evaluate the association of patient characteristics with hypocomplementemia in our large IgG4-RD cohort.
Methods: Patients identified through the Massachusetts General Hospital IgG4-RD cohort were included in this study if they fulfilled the 2019 ACR/EULAR IgG4-RD Classification Criteria or had “probable” IgG4-RD. Probable IgG4-RD is defined as: 1) typical organ involvement; 2) no exclusion criteria present; 3) insufficient total classification points (< 20). The usual scenarios for a diagnosis of probable IgG4-RD are when biopsy is non-diagnostic or not obtained. Hypocomplementemia was defined as any measurement of serum C3 or C4 complement below the lower limit of normal (LLN). Disease characteristics were extracted via review of medical records. Statistical analyses were unpaired T-tests and Chi-squared tests.
Results: Hypocomplementemia was identified in 91 (30%) of 306 patients in the cohort. Characteristics of patients with and without hypocomplementemia are summarized in Table 1. Patients with hypocomplementemia were less likely to have normal serum IgG4 (11% vs. 26%, p=0.009) and more likely to have highly elevated (above 5x the upper limit of normal) serum IgG4 (52% vs. 28%, p=0.001). Patients with hypocomplementemia had more organs involved (mean 4.3 vs. 2.9, p< 0.001). Among patients with hypocomplementemia, C3 was low in 79%, C4 in 84%, and both in 64%. 17% of C3 troughs and 43% of C4 troughs were at or below one-half of the LLN for the assay.
Patients with vs. without hypocomplementemia more commonly had submandibular gland, lymph node, lung, liver, and renal involvement as well as constitutional symptoms (Figure 1). Retroperitoneal involvement was unusual among hypocomplementemic patients. Of the 84 patients with IgG4-related kidney disease (IgG4-RKD), 38 (45%) had hypocomplementemia. Hypocomplementemic patients with IgG4-RKD had significantly more organs involved (mean 5.2 vs. 3.7, p=0.005), more commonly had both low C3 and C4 complement (84% vs. 47%, p=0.03), and more commonly had C3 or C4 troughs at or below one-half of the LLN (24% vs. 6%, p=0.02 for C3; 66% vs. 26%, p=0.003 for C4) than those without IgG4-RKD (Table 2).
Conclusion: Hypocomplementemia is common, and often profound, in IgG4-RD and is associated with serum IgG4 concentration and multiorgan involvement. Additional investigations are needed to describe the nature of complement activation and its role in disease activity, both in the kidneys and beyond.
 Table 1. Characteristics of IgG4-RD patients with and without hypocomplementemia. *IgG4 concentration was not available for one patient without hypocomplementemia. Values are reported as n (%) unless noted otherwise. ULN: upper limit of normal, LLN: lower limit of normal; SD: standard deviation.
Table 1. Characteristics of IgG4-RD patients with and without hypocomplementemia. *IgG4 concentration was not available for one patient without hypocomplementemia. Values are reported as n (%) unless noted otherwise. ULN: upper limit of normal, LLN: lower limit of normal; SD: standard deviation..jpg) Figure 1. IgG4-RD manifestations in patients with and without hypocomplementemia. *Signifies P < 0.05.
Figure 1. IgG4-RD manifestations in patients with and without hypocomplementemia. *Signifies P < 0.05. Table 2. Hypocomplementemic patients with and without IgG4-related kidney disease. Values are reported as n (%) unless noted otherwise. ULN: upper limit of normal, LLN: lower limit of normal; SD: standard deviation.
Table 2. Hypocomplementemic patients with and without IgG4-related kidney disease. Values are reported as n (%) unless noted otherwise. ULN: upper limit of normal, LLN: lower limit of normal; SD: standard deviation.Disclosures: G. Katz, None; A. Cogan, None; G. McMahon, None; S. Perez-Espina, None; A. Fernandes, None; C. Perugino, None; Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Horizon, Shionogi; J. Atkinson, Compliment Corporation, Kypha, Inc., Gemini Therapeutics, Q32 Bio, Celldex Therapeutics, Clinical Pharmacy Services, CDMI, Kairos Bioconsulting, LLC, Achillion Pharmaceuticals, Inc, BioMarin Pharmaceutical Inc., Annexon Biosciences, Inc., Alexion Pharmaceuticals, Broadwing Bio, LLC; A. Kim, GlaxoSmithKline, Kypha Inc., Foghorn Therapeutics, Aurinia Pharmaceuticals, Exagen Diagnostics, Alexion Pharmaceuticals, Pfizer, AstraZeneca; J. Stone, Horizon Theraputics, Sanofi, Amgen, Argenx, Bristol-Myers Squibb(BMS), Chemocentryx, Kyverna, Novartis, Palleon Pharmaceuticals, PPD, Q32, Star Therapeutics, Roche, Mirabio, Spruce Biosciences, Steritas, Zenas.

