Back
Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1004–1034) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster II
1006: Impact of Uric Acid Levels on Clinical and Radiographic Characteristics in Psoriatic Arthritis and Response to Secukinumab: A Pooled “Post Hoc” Analysis from Five Phase 3 Studies
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time

Renaud FELTEN, MD
Hopitaux Universitaires de Strasbourg
STRASBOURG, France
Abstract Poster Presenter(s)
Renaud FELTEN1, Laura Widawski2, Lionel Spielmann2, Corine Gaillez3, Weibin Bao4, Hugh O’Neill5, jacques-eric gottenberg6, Pierre-Marie Duret2 and Laurent Messer7, 1Hôpitaux Universitaires de Strasbourg, Strasbourg, France, 2Colmar Civilian Hospitals, Colmar, France, 3Novartis Pharma AG, Basel, Switzerland, 4Novartis Pharmaceuticals Corporation, East Hanover, NJ, 5Novartis Ireland Limited, Dublin, Ireland, 6Strasbourg University Hospital, Strasbourg, France, 7Colmar Civil Hospitals, Colmar, France
Background/Purpose: Hyperuricemia (HU), a common metabolic abnormality, may result in development of gout, which is an inflammatory arthritic condition1,2. Patients (pts) with psoriasis (PsO)/psoriatic arthritis (PsA) are at increased risk of HU and development of gout1. Moreover, PsA pts with HU respond poorly to PsA treatment and have more peripheral and destructive joint damage3. This post hoc analysis from the FUTURE 2–5 and MAXIMISE studies investigated the impact of different levels of HU, and history of gout/uric acid lowering therapy (ULT) on pts with PsA in terms of demographics, clinical characteristics, comorbidities, and clinical response to secukinumab (SEC) over 1 year.
Methods: Pts were stratified into HU and normouricemic (NU) groups based on baseline (BL) serum uric acid (SUA) threshold of 360 µmol/L and 420 µmol/L. A sensitivity analysis was performed in a subgroup of pts with and without a history of gout/ULT. Demographics, clinical characteristics, van der Heijde modified total sharp score (vdH-mTSS) and comorbidities data were collected at BL. Evaluations included ACR20/50/70, Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis index, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Psoriasis Area and Severity Index (PASI90), Psoriatic Arthritis Response Criteria (PsARC), radiographic structural progression (vdH-mTSS), and quality of life (QoL) up to Week 52 (W52). Analyses were performed at a descriptive level and data are presented as observed.
Results: Overall, 2504 pts with PsA were included in the analysis, of whom 822 (32.8%) and 356 (14.3%) had SUA level ≥ 360 and ≥ 420 µmol/L, respectively, and 62 (2.5%) had a history of gout/ULT. At BL, HU pts (SUA ≥ 360 and ≥ 420 µmol/L) and pts with a history of gout/ULT were mostly male, had higher body mass index with frequent hypertension. Pts with SUA ≥ 420 µmol/L appeared to have higher vdH-mTSS score vs NU pts (Table 1). At W52, efficacy response as measured by ACR20/50/70, SPARCC enthesitis index, BASDAI, PsARC, vdH-mTSS score (Figure 1), and improvement in QoL was similar with SEC 150 and 300 mg, irrespective of BL HU status (Table 2). In the subset of pts with PsO, a higher proportion of HU pts had moderate to severe PsO, compared with NU pts. As a result, higher PASI90 response was observed in NU pts with SEC 150 mg (with and without load). However, PASI90 response was comparable with SEC 300 mg irrespective of BL HU status (Table 2).
Conclusion: Pts with HU and a history of gout/ULT had a higher prevalence of hypertension, and more prominent clinical presentation of moderate to severe PsO and dactylitis. Efficacy across all musculoskeletal manifestations was similar with SEC 150 and 300 mg up to W52. At BL, HU pts (≥ 420 µmol/L) appeared to have higher vdH-mTSS scores with large variability than NU pts; however, responses to SEC were similar at W52, preventing radiographic progression. Although these findings were not consistent in pts with SUA ≥ 360 µmol/L or gout/ULT history, further investigations are needed to confirm the impact of urate on the structural damage in PsA.
References:
Tripolino C, et al. Front Med. 2021;8:737573 Keenan R. Clin Ther. 2017;39(2):430‒41 Widawski L, et al. Clin Rheumatol. 2022;41:1421‒29
.jpg) Table 1. Demographics and clinical characteristics
Table 1. Demographics and clinical characteristics
.jpg) Table 2. Clinical efficacy outcomes at Week 52
Table 2. Clinical efficacy outcomes at Week 52
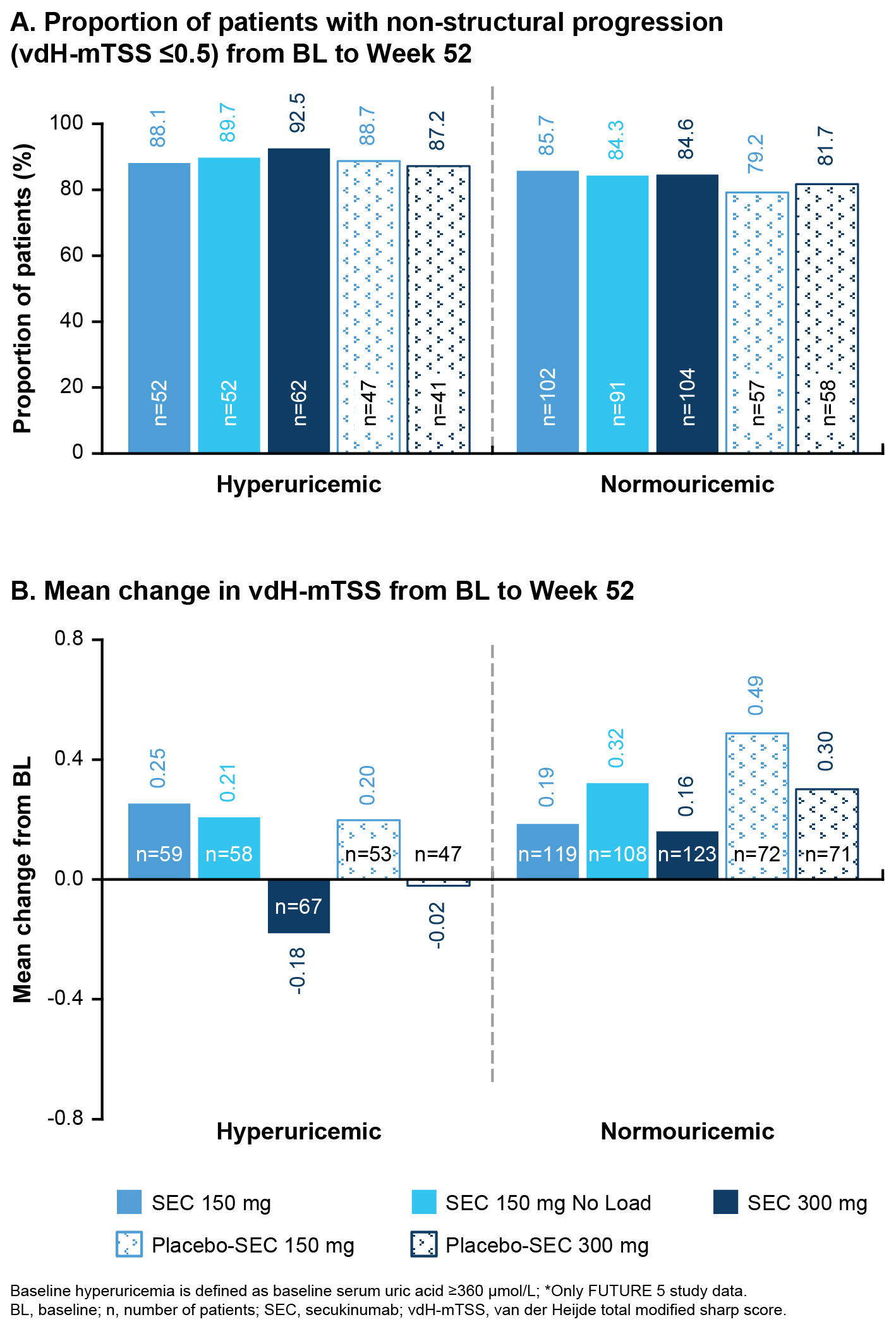 Figure 1: Assessment of inhibition of structural damage progression
Figure 1: Assessment of inhibition of structural damage progression
Disclosures: R. FELTEN, Novartis, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Nordic Pharma, Merck/MSD, MEDAC, Pfizer, Sanofi, UCB; L. Widawski, None; L. Spielmann, None; C. Gaillez, Novartis; W. Bao, Novartis; H. O’Neill, Novartis; j. gottenberg, AbbVie, Bristol Myers Squibb, Galapagos, Gilead, Lilly, MSD, Novartis, Pfizer; P. Duret, None; L. Messer, None.
Background/Purpose: Hyperuricemia (HU), a common metabolic abnormality, may result in development of gout, which is an inflammatory arthritic condition1,2. Patients (pts) with psoriasis (PsO)/psoriatic arthritis (PsA) are at increased risk of HU and development of gout1. Moreover, PsA pts with HU respond poorly to PsA treatment and have more peripheral and destructive joint damage3. This post hoc analysis from the FUTURE 2–5 and MAXIMISE studies investigated the impact of different levels of HU, and history of gout/uric acid lowering therapy (ULT) on pts with PsA in terms of demographics, clinical characteristics, comorbidities, and clinical response to secukinumab (SEC) over 1 year.
Methods: Pts were stratified into HU and normouricemic (NU) groups based on baseline (BL) serum uric acid (SUA) threshold of 360 µmol/L and 420 µmol/L. A sensitivity analysis was performed in a subgroup of pts with and without a history of gout/ULT. Demographics, clinical characteristics, van der Heijde modified total sharp score (vdH-mTSS) and comorbidities data were collected at BL. Evaluations included ACR20/50/70, Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis index, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Psoriasis Area and Severity Index (PASI90), Psoriatic Arthritis Response Criteria (PsARC), radiographic structural progression (vdH-mTSS), and quality of life (QoL) up to Week 52 (W52). Analyses were performed at a descriptive level and data are presented as observed.
Results: Overall, 2504 pts with PsA were included in the analysis, of whom 822 (32.8%) and 356 (14.3%) had SUA level ≥ 360 and ≥ 420 µmol/L, respectively, and 62 (2.5%) had a history of gout/ULT. At BL, HU pts (SUA ≥ 360 and ≥ 420 µmol/L) and pts with a history of gout/ULT were mostly male, had higher body mass index with frequent hypertension. Pts with SUA ≥ 420 µmol/L appeared to have higher vdH-mTSS score vs NU pts (Table 1). At W52, efficacy response as measured by ACR20/50/70, SPARCC enthesitis index, BASDAI, PsARC, vdH-mTSS score (Figure 1), and improvement in QoL was similar with SEC 150 and 300 mg, irrespective of BL HU status (Table 2). In the subset of pts with PsO, a higher proportion of HU pts had moderate to severe PsO, compared with NU pts. As a result, higher PASI90 response was observed in NU pts with SEC 150 mg (with and without load). However, PASI90 response was comparable with SEC 300 mg irrespective of BL HU status (Table 2).
Conclusion: Pts with HU and a history of gout/ULT had a higher prevalence of hypertension, and more prominent clinical presentation of moderate to severe PsO and dactylitis. Efficacy across all musculoskeletal manifestations was similar with SEC 150 and 300 mg up to W52. At BL, HU pts (≥ 420 µmol/L) appeared to have higher vdH-mTSS scores with large variability than NU pts; however, responses to SEC were similar at W52, preventing radiographic progression. Although these findings were not consistent in pts with SUA ≥ 360 µmol/L or gout/ULT history, further investigations are needed to confirm the impact of urate on the structural damage in PsA.
References:
.jpg) Table 1. Demographics and clinical characteristics
Table 1. Demographics and clinical characteristics.jpg) Table 2. Clinical efficacy outcomes at Week 52
Table 2. Clinical efficacy outcomes at Week 52 Figure 1: Assessment of inhibition of structural damage progression
Figure 1: Assessment of inhibition of structural damage progressionDisclosures: R. FELTEN, Novartis, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Nordic Pharma, Merck/MSD, MEDAC, Pfizer, Sanofi, UCB; L. Widawski, None; L. Spielmann, None; C. Gaillez, Novartis; W. Bao, Novartis; H. O’Neill, Novartis; j. gottenberg, AbbVie, Bristol Myers Squibb, Galapagos, Gilead, Lilly, MSD, Novartis, Pfizer; P. Duret, None; L. Messer, None.

