Back
Poster Session B
Juvenile idiopathic arthritis (JIA) and pediatric joint disorders
Session: (0850–0880) Pediatric Rheumatology – Clinical Poster I: JIA
0872: Incidence, Risk Factors, and Outcomes of Eosinophilia on IL-1 and IL-6 Inhibitors in Systemic and Non-Systemic Juvenile Idiopathic Arthritis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- HW
Holly Wobma, MD, PhD
Boston Children's Hospital
Boston, MA, United States
Abstract Poster Presenter(s)
Holly Wobma1, Maria Taylor2, Ki Pui Lam1, Helene Brown1, Siobhan Case1, Mia Chandler1, Margaret Chang1, Ezra Cohen1, Megan Day-Lewis1, Olha Halyabar1, Jonathan Hausmann1, Melissa Hazen3, Erin Janssen1, Pui Lee1, Mindy Lo1, Esra Meidan1, Jordan Roberts1, Mary Beth F. Son1, Robert Sundel1, Fatma Dedeoglu1, Peter Nigrovic1, Alicia Casey4, Joyce Chang1 and lauren henderson1, 1Division of Immunology, Boston Children's Hospital, Boston, MA, 2Division of Immunology, Boston Children's Hospital, Brighton, MA, 3Boston Children's Hospital, Boston, MA, 4Division of Pulmonary Medicine, Boston Children's Hospital, Boston, MA
Background/Purpose: Children with systemic juvenile idiopathic arthritis (sJIA) exposed to IL1/6 inhibitors may develop eosinophilia with an atypical rash, reportedly in association with HLA-DRB1*15:XX. There is concern that these features, reminiscent of a drug reaction, may drive macrophage activation syndrome (MAS) and sJIA-lung disease (LD). We estimated rates and outcomes of eosinophilia on biologic therapy in sJIA patients compared to non-systemic forms of JIA by drug class and HLA type.
Methods: We reviewed JIA patients of all ILAR subtypes exposed to IL1/6 inhibitors at our center (1995-2022). HLA typing was performed on a clinical (n=10) or research basis (n=26 in our biobank). We compared time exposed to IL1/6 inhibitors to time on TNF inhibitor/other biologics and methotrexate in the same cohort. Exposure time was defined as drug start date to first eosinophilia event on drug or censoring (drug stop or last follow-up). Eosinophilia was defined as 2 consecutive absolute eosinophil counts (AEC) >500/μL or doubling of baseline AEC. We identified predictors of eosinophilia in IL1/6 inhibitor-exposed sJIA patients using Cox models. Features at time of eosinophilia were compared to those in patients without eosinophilia. We summarized changes in treatment and disease activity within 10 weeks of developing eosinophilia. A pulmonologist adjudicated LD cases.
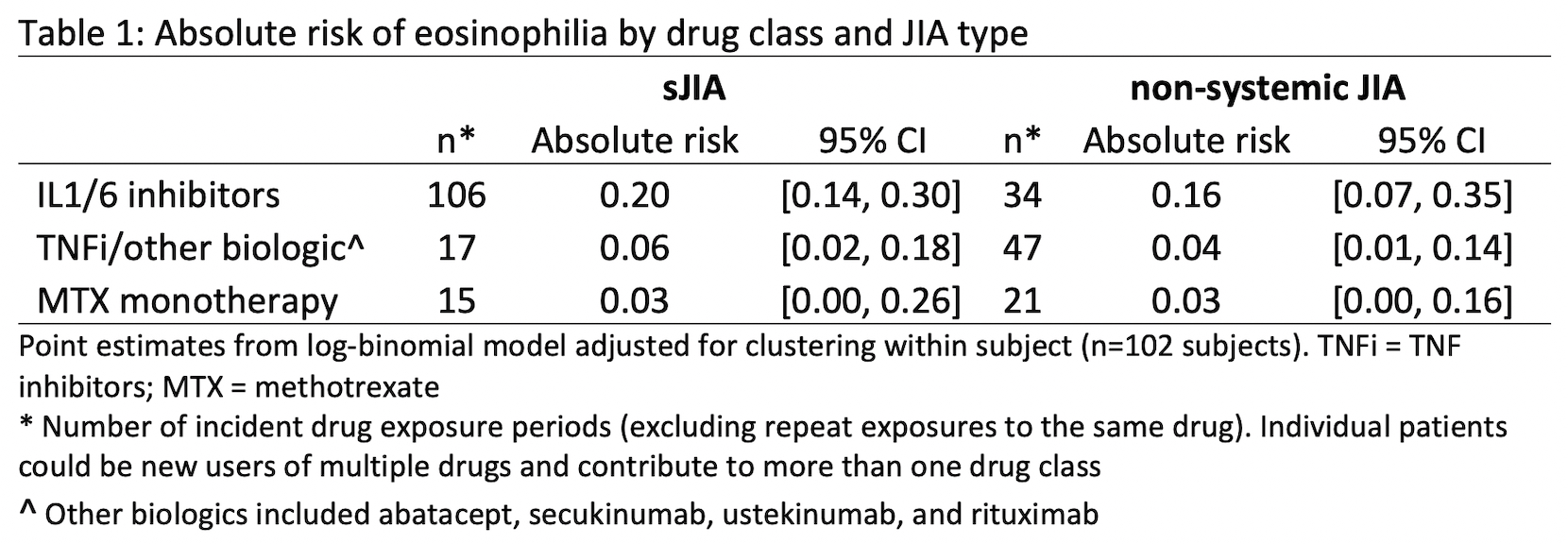
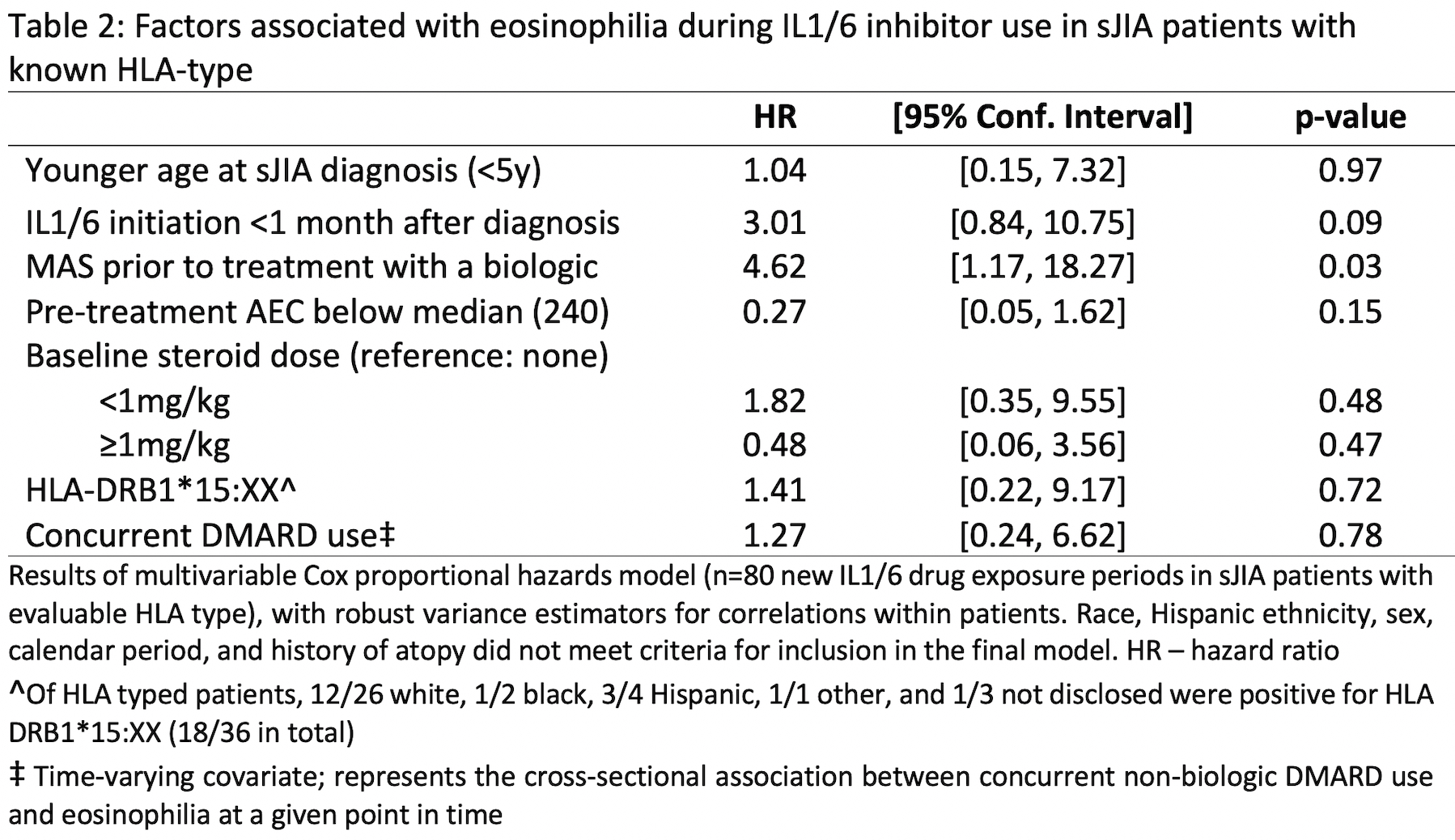
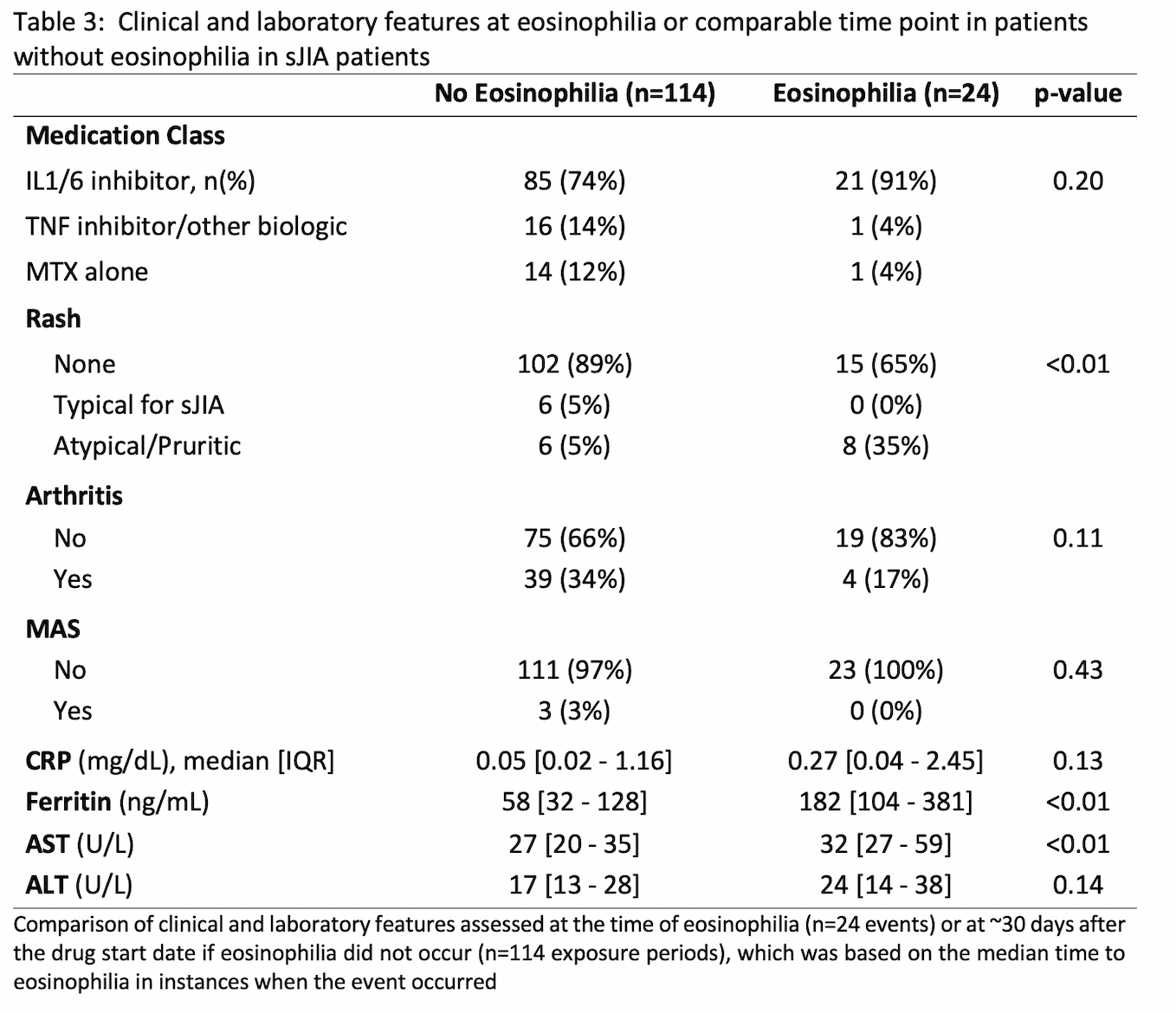
Results: There were 240 new drug exposures for 67 (sJIA) and 36 (non-systemic JIA) patients with median follow-up of 70 months [IQR 36-135]. Eosinophilia was common during IL1/6 inhibitor use in both groups at 0.10/person-yrs (absolute risks shown in Table 1) and did not differ by DRB*15:XX status (positive in 18/36 typed). For sJIA patients on IL1/6 inhibitors, the only significant predictor of eosinophilia was MAS prior to treatment with a biologic (Table 2). At the time of eosinophilia, sJIA patients on IL1/6 inhibitors had well-controlled disease and there were no instances of MAS (Table 3). Of 23 eosinophilia events in sJIA patients during IL1/6 inhibitor use, 5 switched biologics, resulting in disease flare/MAS in 4/5. Eosinophilia normalized upon adding a non-biologic DMARD in 5/7 events without worsening disease activity. Of 21 sJIA patients referred for LD evaluation, there were 9 confirmed cases of varying severity. Preceding eosinophilia and DRB1*15:XX positivity were prevalent in LD cases (6/9 and 5/6, respectively); having one of these factors was also strongly associated with referral for LD evaluation (p=0.02). Two patients had severely progressive LD; neither had preceding eosinophilia, and LD preceded IL1/6 inhibitor use in one. Of 46 patients not referred for LD evaluation, 20% had eosinophilia and 41% had the DRB1*15:XX allele; all remained free of significant respiratory signs/symptoms over a median of 57 months [IQR 26-108].
Conclusion: Eosinophilia is common in JIA patients using IL1/6 inhibitors and may be associated with pre-treatment MAS in sJIA. Switching biologics due to isolated eosinophilia should be considered carefully as it precipitated disease flare in our cohort. Additional risk factors for sJIA-LD remain to be defined, as eosinophilia and DRB1*15:XX alleles were neither necessary nor sufficient for LD in our sJIA patients.
Disclosures: H. Wobma, Immplacate Inc; M. Taylor, None; K. Lam, None; H. Brown, None; S. Case, None; M. Chandler, None; M. Chang, None; E. Cohen, Cornerstone Research Group, Mend Health; M. Day-Lewis, None; O. Halyabar, None; J. Hausmann, Novartis, Pfizer, Biogen; M. Hazen, None; E. Janssen, None; P. Lee, None; M. Lo, GlaxoSmithKlein(GSK); E. Meidan, None; J. Roberts, None; M. Son, None; R. Sundel, None; F. Dedeoglu, Novartis; P. Nigrovic, Bristol-Myers Squibb(BMS), Exo Therapeutics, Miach Orthopedics, Pfizer, Novartis, UpToDate, Cerecor, Brickell Bio; A. Casey, None; J. Chang, GlaxoSmithKline (GSK); l. henderson, Sobi, Pfizer, Adaptive Biotechnologies, BMS.
Background/Purpose: Children with systemic juvenile idiopathic arthritis (sJIA) exposed to IL1/6 inhibitors may develop eosinophilia with an atypical rash, reportedly in association with HLA-DRB1*15:XX. There is concern that these features, reminiscent of a drug reaction, may drive macrophage activation syndrome (MAS) and sJIA-lung disease (LD). We estimated rates and outcomes of eosinophilia on biologic therapy in sJIA patients compared to non-systemic forms of JIA by drug class and HLA type.
Methods: We reviewed JIA patients of all ILAR subtypes exposed to IL1/6 inhibitors at our center (1995-2022). HLA typing was performed on a clinical (n=10) or research basis (n=26 in our biobank). We compared time exposed to IL1/6 inhibitors to time on TNF inhibitor/other biologics and methotrexate in the same cohort. Exposure time was defined as drug start date to first eosinophilia event on drug or censoring (drug stop or last follow-up). Eosinophilia was defined as 2 consecutive absolute eosinophil counts (AEC) >500/μL or doubling of baseline AEC. We identified predictors of eosinophilia in IL1/6 inhibitor-exposed sJIA patients using Cox models. Features at time of eosinophilia were compared to those in patients without eosinophilia. We summarized changes in treatment and disease activity within 10 weeks of developing eosinophilia. A pulmonologist adjudicated LD cases.
Results: There were 240 new drug exposures for 67 (sJIA) and 36 (non-systemic JIA) patients with median follow-up of 70 months [IQR 36-135]. Eosinophilia was common during IL1/6 inhibitor use in both groups at 0.10/person-yrs (absolute risks shown in Table 1) and did not differ by DRB*15:XX status (positive in 18/36 typed). For sJIA patients on IL1/6 inhibitors, the only significant predictor of eosinophilia was MAS prior to treatment with a biologic (Table 2). At the time of eosinophilia, sJIA patients on IL1/6 inhibitors had well-controlled disease and there were no instances of MAS (Table 3). Of 23 eosinophilia events in sJIA patients during IL1/6 inhibitor use, 5 switched biologics, resulting in disease flare/MAS in 4/5. Eosinophilia normalized upon adding a non-biologic DMARD in 5/7 events without worsening disease activity. Of 21 sJIA patients referred for LD evaluation, there were 9 confirmed cases of varying severity. Preceding eosinophilia and DRB1*15:XX positivity were prevalent in LD cases (6/9 and 5/6, respectively); having one of these factors was also strongly associated with referral for LD evaluation (p=0.02). Two patients had severely progressive LD; neither had preceding eosinophilia, and LD preceded IL1/6 inhibitor use in one. Of 46 patients not referred for LD evaluation, 20% had eosinophilia and 41% had the DRB1*15:XX allele; all remained free of significant respiratory signs/symptoms over a median of 57 months [IQR 26-108].
Conclusion: Eosinophilia is common in JIA patients using IL1/6 inhibitors and may be associated with pre-treatment MAS in sJIA. Switching biologics due to isolated eosinophilia should be considered carefully as it precipitated disease flare in our cohort. Additional risk factors for sJIA-LD remain to be defined, as eosinophilia and DRB1*15:XX alleles were neither necessary nor sufficient for LD in our sJIA patients.



Disclosures: H. Wobma, Immplacate Inc; M. Taylor, None; K. Lam, None; H. Brown, None; S. Case, None; M. Chandler, None; M. Chang, None; E. Cohen, Cornerstone Research Group, Mend Health; M. Day-Lewis, None; O. Halyabar, None; J. Hausmann, Novartis, Pfizer, Biogen; M. Hazen, None; E. Janssen, None; P. Lee, None; M. Lo, GlaxoSmithKlein(GSK); E. Meidan, None; J. Roberts, None; M. Son, None; R. Sundel, None; F. Dedeoglu, Novartis; P. Nigrovic, Bristol-Myers Squibb(BMS), Exo Therapeutics, Miach Orthopedics, Pfizer, Novartis, UpToDate, Cerecor, Brickell Bio; A. Casey, None; J. Chang, GlaxoSmithKline (GSK); l. henderson, Sobi, Pfizer, Adaptive Biotechnologies, BMS.

