Back
Poster Session B
Session: (0807–0832) Miscellaneous Rheumatic and Inflammatory Diseases Poster II
0816: Increased Risk of Hepatotoxicity with DMARDS in Patients with Previous Liver Toxicity with Isoniazid: Study in a Single University Hospital
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- DM
David Martinez-Lopez, MD
Hospital Universitario Marques de Valdecilla
Santander (SPAIN), Spain
Abstract Poster Presenter(s)
David Martínez-López1, Joy Osorio-Chávez1, Virgi Portilla2, Carmen Alvarez Reguera1, Alba Herrero-Morant3, Lara Sánchez-Bilbao1, iñigo Gonzalez-Mazon1, Miguel Ángel González-Gay4 and Ricardo Blanco5, 1Hospital Universitario Marqués de Valdecilla, Santander, Spain, 2Research Group on Genetic Epidemiology and Atherosclerosis in Systemic Diseases and in Metabolic Bone Diseases of the Musculoskeletal System, IDIVAL; and Department of Rheumatology, Hospital Universitario Marqués de Valdecilla, Santander, Spain, 3Hospital Universitario Marqués de Valdecilla, Ontinyent, Spain, 4Department of Medicine and Psychiatry, Universidad de Cantabria; Rheumatology Division, Hospital Universitario Marqués de Valdecilla; Research group on genetic epidemiology and atherosclerosis in systemic diseases and in metabolic diseases of the musculoskeletal system, IDIVAL, Santander, Spain. Cardiovascular Pathophysiology and Genomics Research Unit, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa, 5Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain
Background/Purpose: Isoniazid (INH) is used to treat latent tuberculosis infection (LTBI), and hepatotoxicity is one of the most frequent adverse effect. Several Disease-modifying drugs (DMARDs) can also cause hepatotoxicity. Many patients with rheumatic immune mediated diseases (R-IMID) receive INH prior to DMARDs for prophylaxis of LTBI. This risk of hepatotoxicity with DMARDs after hepatotoxicity with INH is unknown.
Our aim was to assess the risk of hepatotoxicity with DMARDs in patients who have presented hepatotoxicity with INH.
Methods: Study of all consecutive R-IMID patients evaluated in the last five years (2016-2020) in a University Hospital, who presented hepatotoxicity after INH and later received DMARDs. We study if they also presented hepatotoxicity with DMARDs. Hepatotoxicity was defined as an elevation of liver enzymes (ALT and/or AST) upper the high limit after the introduction of the treatment.
Results: INH was used in 232 of 7218 patients with R-IMID. We finally included 64 patients (45 women; 70.3%; mean age 53.4±10.5 years), who had hepatotoxicity due to INH (TABLE). The most frequent R-IMIDs were rheumatoid arthritis, axial spondyloarthritis and psoriatic arthritis. Methotrexate (MTX) (n=34, 53.1%) and TNF inhibitors (n=27, 42.2%) were the conventional and biologic-DMARD more frequently used, respectively. Hepatotoxicity was higher with MTX (14 of 34, 41.2%), and lower with the other DMARDs (FIGURE). Hepatotoxicity was not observed with hydroxychloroquine, azathioprine, mycophenolate mofetil, secukinumab, abatacept or rituximab.
Conclusion: In patients with previous hepatotoxicity with INH, we observed an increased risk with different DMARDs, especially with MTX.
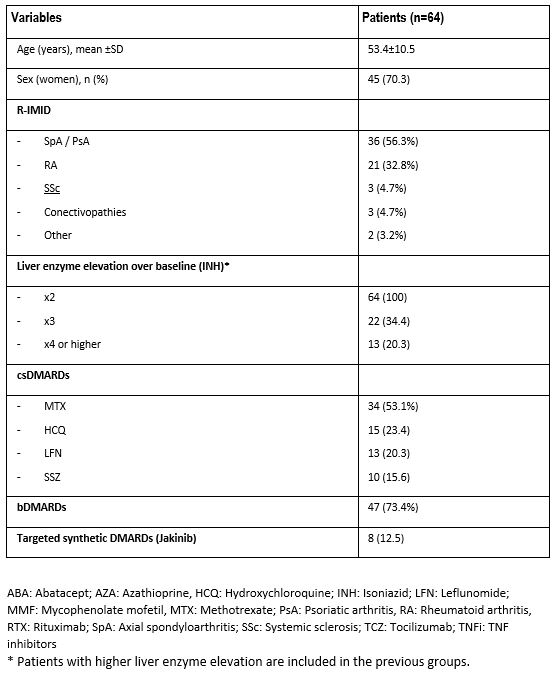 TABLE. Main characteristics of 64 patients with rheumatic immune-mediated diseases (R-IMID) that presented hepatotoxicity after receiving isoniazid (INH).
TABLE. Main characteristics of 64 patients with rheumatic immune-mediated diseases (R-IMID) that presented hepatotoxicity after receiving isoniazid (INH).
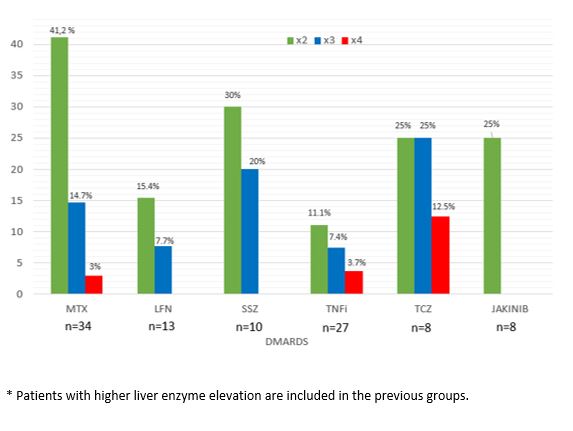 FIGURE: Hepatotoxicity with different DMARDs in 64 patients with previous hepatotoxicity with INH
FIGURE: Hepatotoxicity with different DMARDs in 64 patients with previous hepatotoxicity with INH
Disclosures: D. Martínez-López, None; J. Osorio-Chávez, None; V. Portilla, None; C. Alvarez Reguera, None; A. Herrero-Morant, None; L. Sánchez-Bilbao, Eli Lilly; i. Gonzalez-Mazon, None; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.
Background/Purpose: Isoniazid (INH) is used to treat latent tuberculosis infection (LTBI), and hepatotoxicity is one of the most frequent adverse effect. Several Disease-modifying drugs (DMARDs) can also cause hepatotoxicity. Many patients with rheumatic immune mediated diseases (R-IMID) receive INH prior to DMARDs for prophylaxis of LTBI. This risk of hepatotoxicity with DMARDs after hepatotoxicity with INH is unknown.
Our aim was to assess the risk of hepatotoxicity with DMARDs in patients who have presented hepatotoxicity with INH.
Methods: Study of all consecutive R-IMID patients evaluated in the last five years (2016-2020) in a University Hospital, who presented hepatotoxicity after INH and later received DMARDs. We study if they also presented hepatotoxicity with DMARDs. Hepatotoxicity was defined as an elevation of liver enzymes (ALT and/or AST) upper the high limit after the introduction of the treatment.
Results: INH was used in 232 of 7218 patients with R-IMID. We finally included 64 patients (45 women; 70.3%; mean age 53.4±10.5 years), who had hepatotoxicity due to INH (TABLE). The most frequent R-IMIDs were rheumatoid arthritis, axial spondyloarthritis and psoriatic arthritis. Methotrexate (MTX) (n=34, 53.1%) and TNF inhibitors (n=27, 42.2%) were the conventional and biologic-DMARD more frequently used, respectively. Hepatotoxicity was higher with MTX (14 of 34, 41.2%), and lower with the other DMARDs (FIGURE). Hepatotoxicity was not observed with hydroxychloroquine, azathioprine, mycophenolate mofetil, secukinumab, abatacept or rituximab.
Conclusion: In patients with previous hepatotoxicity with INH, we observed an increased risk with different DMARDs, especially with MTX.
 TABLE. Main characteristics of 64 patients with rheumatic immune-mediated diseases (R-IMID) that presented hepatotoxicity after receiving isoniazid (INH).
TABLE. Main characteristics of 64 patients with rheumatic immune-mediated diseases (R-IMID) that presented hepatotoxicity after receiving isoniazid (INH). FIGURE: Hepatotoxicity with different DMARDs in 64 patients with previous hepatotoxicity with INH
FIGURE: Hepatotoxicity with different DMARDs in 64 patients with previous hepatotoxicity with INH Disclosures: D. Martínez-López, None; J. Osorio-Chávez, None; V. Portilla, None; C. Alvarez Reguera, None; A. Herrero-Morant, None; L. Sánchez-Bilbao, Eli Lilly; i. Gonzalez-Mazon, None; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.

