Back
Abstract Session
Session: Abstracts: RA – Treatment III: Comorbidities and Consequences (2215–2220)
2216: JAK Inhibitors and Risk of Cancer
Monday, November 14, 2022
3:15 PM – 3:25 PM Eastern Time
Location: Exhibit Hall A

Jérôme Avouac, MD, PhD
Université de Paris Cité
Paris, France
Presenting Author(s)
Amandine Gouverneur1, Jérôme Avouac2, Clément Prati3, Jean-Luc Cracowski4, Thierry schaeverbeke5, Antoine Pariente1 and Marie-Elise Truchetet5, 1Université de Bordeaux, Bordeaux, France, 2University of Paris, Paris, France, 3Service de rhumatologie, CHU de Besançon, Besançon, France, 4Université de Grenoble, Grenoble, France, 5CHU de Bordeaux, Bordeaux, France
Background/Purpose: Recent concerns have been raised about potential increase of cancer risk under JAK inhibitors (JAKi) especially tofacitinib. Discrepant findings have been given by randomized controlled trials and registries. This study aims at assess the risk of cancer with the use of JAKi in the French health database.
Methods: A propensity-score matching cohort analysis was performed using data from the French national healthcare insurance system SNDS ("Système National des Données de Santé"), which included all anonymized individual level data about sociodemographic data, outpatient healthcare dispensed, hospital discharge summaries, and registration status for a list of 30 long term diseases. Among all patients with rheumatoid arthritis between 1st November 2017 and 30 June 2019, patients newly treated by JAKi or tumor necrosis factor inhibitors (TNF-i) have been included (absence of any JAKi or TNFi dispensing in the previous year). The first dispensing of JAKi or TNFi constituted the index date and cancer events were identified at least 6 months after the index date. Thereafter, a Cox regression model stratified on the matching ratio and adjusted on covariates that remained unbalanced after propensity score matching (standardized difference >0.1) was performed.
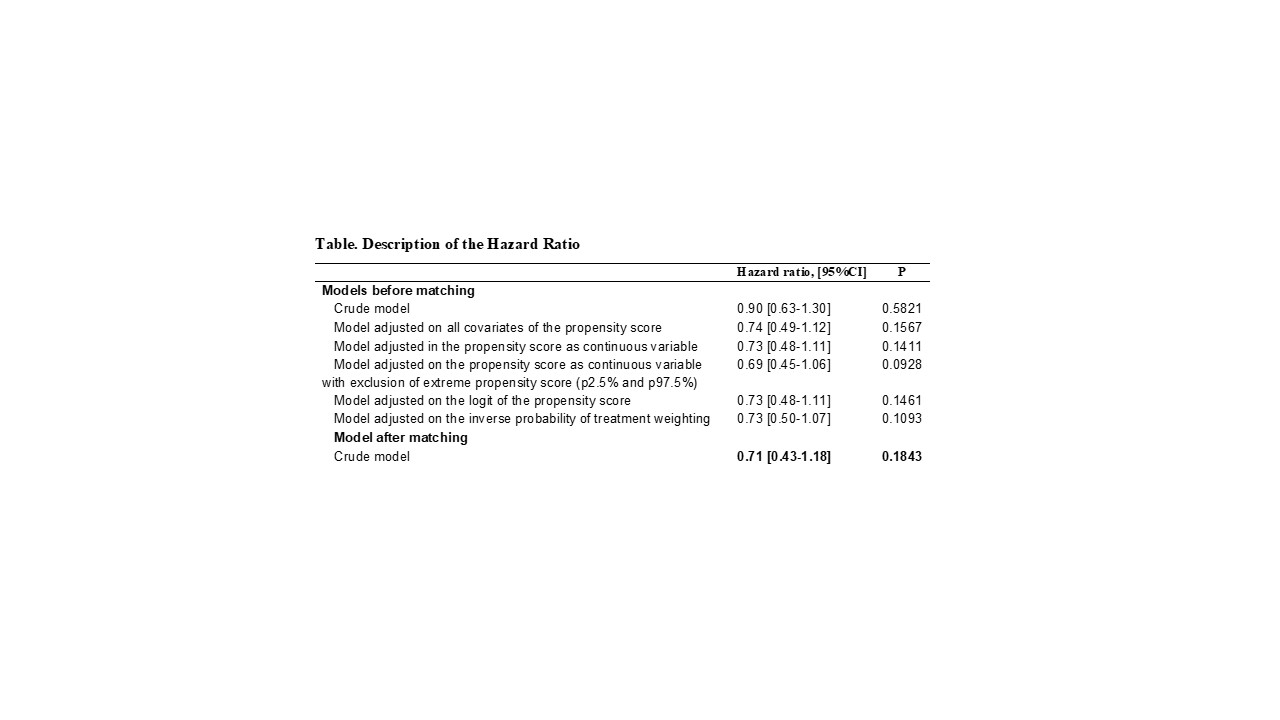
Results: Overall 3132 (67.4% of identified patients) patients treated by JAKi were matched to 3132 (55.2%) patients treated by TNFi. After matching, all characteristics of interest were well balanced between both groups. Among the overall matched population, 64 (1.0%) patients had a diagnosis of cancer, 25 (0.8%) treated with JAKi, (mainly melanoma (n=7, 28.0%), lung cancer (n=5, 20.0%) and breast cancer (n=5, 20.0%)) and 39 (1.3%) treated with TNFi (mainly hematologic cancer (n=12, 30.8%), prostate cancer (n=5, 12.8%), melanoma (n=4, 10.3%), and pancreas cancer (n=4, 10.3%)). The median time-to-onset was 12.6 months IQR [9.8-13.8] for patients treated with JAKi and 10.3 months IQR [7.7-13.6] for patients treated with TNFi. In the multivariate analyses, we did not find any significant difference between patients treated by JAKi and those treated by TNFi (HR: 0.71, 95% CI 0.43-1.18).
Conclusion: In this nationwide population-based study, exposure to JAKi was not found to be associated with a higher risk of cancer in comparison to exposure of TNFi. These important data should be complemented by careful monitoring over time.
 Table
Table
Disclosures: A. Gouverneur, None; J. Avouac, Galapagos, AbbVie, Lilly, Pfizer, Bristol Myers Squibb, Novartis, Fresenius-Kabi, Sanofi, Sandoz, Nordic Pharma, Biogen, Medac, Janssen, Roche-Chugai; C. Prati, None; J. Cracowski, None; T. schaeverbeke, None; A. Pariente, ANSM; M. Truchetet, AbbVie/Abbott, Galapagos, Eli Lilly, Medac, Novartis, Pfizer, Roche.
Background/Purpose: Recent concerns have been raised about potential increase of cancer risk under JAK inhibitors (JAKi) especially tofacitinib. Discrepant findings have been given by randomized controlled trials and registries. This study aims at assess the risk of cancer with the use of JAKi in the French health database.
Methods: A propensity-score matching cohort analysis was performed using data from the French national healthcare insurance system SNDS ("Système National des Données de Santé"), which included all anonymized individual level data about sociodemographic data, outpatient healthcare dispensed, hospital discharge summaries, and registration status for a list of 30 long term diseases. Among all patients with rheumatoid arthritis between 1st November 2017 and 30 June 2019, patients newly treated by JAKi or tumor necrosis factor inhibitors (TNF-i) have been included (absence of any JAKi or TNFi dispensing in the previous year). The first dispensing of JAKi or TNFi constituted the index date and cancer events were identified at least 6 months after the index date. Thereafter, a Cox regression model stratified on the matching ratio and adjusted on covariates that remained unbalanced after propensity score matching (standardized difference >0.1) was performed.
Results: Overall 3132 (67.4% of identified patients) patients treated by JAKi were matched to 3132 (55.2%) patients treated by TNFi. After matching, all characteristics of interest were well balanced between both groups. Among the overall matched population, 64 (1.0%) patients had a diagnosis of cancer, 25 (0.8%) treated with JAKi, (mainly melanoma (n=7, 28.0%), lung cancer (n=5, 20.0%) and breast cancer (n=5, 20.0%)) and 39 (1.3%) treated with TNFi (mainly hematologic cancer (n=12, 30.8%), prostate cancer (n=5, 12.8%), melanoma (n=4, 10.3%), and pancreas cancer (n=4, 10.3%)). The median time-to-onset was 12.6 months IQR [9.8-13.8] for patients treated with JAKi and 10.3 months IQR [7.7-13.6] for patients treated with TNFi. In the multivariate analyses, we did not find any significant difference between patients treated by JAKi and those treated by TNFi (HR: 0.71, 95% CI 0.43-1.18).
Conclusion: In this nationwide population-based study, exposure to JAKi was not found to be associated with a higher risk of cancer in comparison to exposure of TNFi. These important data should be complemented by careful monitoring over time.
 Table
TableDisclosures: A. Gouverneur, None; J. Avouac, Galapagos, AbbVie, Lilly, Pfizer, Bristol Myers Squibb, Novartis, Fresenius-Kabi, Sanofi, Sandoz, Nordic Pharma, Biogen, Medac, Janssen, Roche-Chugai; C. Prati, None; J. Cracowski, None; T. schaeverbeke, None; A. Pariente, ANSM; M. Truchetet, AbbVie/Abbott, Galapagos, Eli Lilly, Medac, Novartis, Pfizer, Roche.

