Back
Abstract Session
Session: Abstracts: Cognition and Behavior in RA and Systemic Sclerosis (2187–2190)
2188: The Effect of Group-based Cognitive Behavioral Therapy for Insomnia in People with Rheumatoid Arthritis: A Randomized Controlled Trial
Monday, November 14, 2022
3:15 PM – 3:25 PM Eastern Time
Location: Ballroom AB
- BE
Bente Esbensen, RN, PhD, MSN
Rigshospitalet
Glostrup, Denmark
Presenting Author(s)
Kristine Latocha1, Katrine Loeppenthin2, Mikkel Østergaard3, poul jennum4, Merete L Hetland5, Henrik Rogind6, Tine Lundbak6, Julie Midtgaard7, Robin Christensen8 and Bente Esbensen5, 1Copenhagen University Hospital - Rigshospitalet, Glostrup, Denmark, 2Department of Oncology, Copenhagen University Hospital - Rigshospitalet, Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark, 3Rigshospitalet, University of Copenhagen, Glostrup, Denmark, 4Danish Center for Sleep Medicine, Department of Clinical Neurophysiology, Copenhagen University Hospital - Rigshospitalet, Glostrup,Department of Clinical Medicine, University of Copenhagen, Copenhagen, 5Rigshospitalet, Glostrup, Denmark, 6Center for Rheumatology and Spine Diseases, Copenhagen University Hospital -Rigshospitalet, Glostrup, Denmark, 7Mental Health Centre Glostrup, University of Copenhagen, Glostrup,Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark, 8Musculoskeletal Statistics Unit, The Parker Institute, Copenhagen, Denmark
Background/Purpose: Insomnia is highly prevalent in people with rheumatoid arthritis (RA) and may exacerbate symptoms and burdens, such as fatigue, depressive symptoms, and pain (1). Cognitive behavioral therapy for insomnia (CBT-I) has been shown to produce positive effects on sleep in other clinical populations (2,3). However, CBT-I has not previously been investigated in people with RA (1).
The primary objective was to compare the effect of nurse-led group-based CBT-I to usual care on sleep efficiency, measured by polysomnography (PSG) immediately after the intervention (i.e. seven weeks after baseline) in people with RA. Secondary objectives included comparing the longer-term effect of CBT-I on sleep and RA-related outcomes at 26 weeks' follow-up.
Methods: In a randomized controlled trial (ClinicalTrials.gov Identifier NCT03766100), using a parallel-group design, the experimental intervention was six weeks of CBT-I; the control comparator was usual care. CBT-I was delivered face-to-face by a CBT-I trained nurse. The primary analyses were based on the intention-to-treat (ITT) population; missing data were statistically handled using repeated-measures linear mixed-effects models adjusted for the level at baseline.
Results: The ITT population consisted of 62 participants (89% women), with an average age of 58 years (SD 10), DAS28-CRP of 3.4 (SD 1.0), Insomnia Severity Index (ISI) score of 18.9 (SD 4.4) and median Patient Global Assessment score of 55 (IQR 28;71).
When the primary outcome was measured by PSG at week seven, sleep efficiency was 88.7% in the CBT-I group, compared to 83.7% in the control group (difference: 5.0 [95% CI -0.4 to 10.4]; p=0.068) (See Table 1). Secondary outcomes measured by PSG had not improved at week 26 either.
However, for all secondary sleep and RA-related patient-reported outcomes, there were statistically highly significant differences between CBT-I and usual care e.g. Insomnia Severity Index (ISI, range 0-28, higher is worse): -9.8 [95% CI -11.8 to -7.9], RA impact of disease (RAID, range 0-10, higher is worse) -1.4 [95% CI-1.9 to -0.80] and Patient Global Assessment (range 0-100, higher is worse): -13.0 [95% CI -20.9 to -5.1] at 26 weeks' follow-up.
Conclusion: Nurse-led, group-based CBT-I for two hours per week for six weeks, did not improve objectively measured sleep efficiency or any other outcomes measured by PSG. However, CBT-I showed long-term improvement on patient-reported outcomes such as fatigue, impact of disease, depression, pain, and Patient Global Assessment – a finding that could have important clinical implications.
References
(1) PMID: 25620673
(2) PMID: 16804151
(3) PMID: 26434673
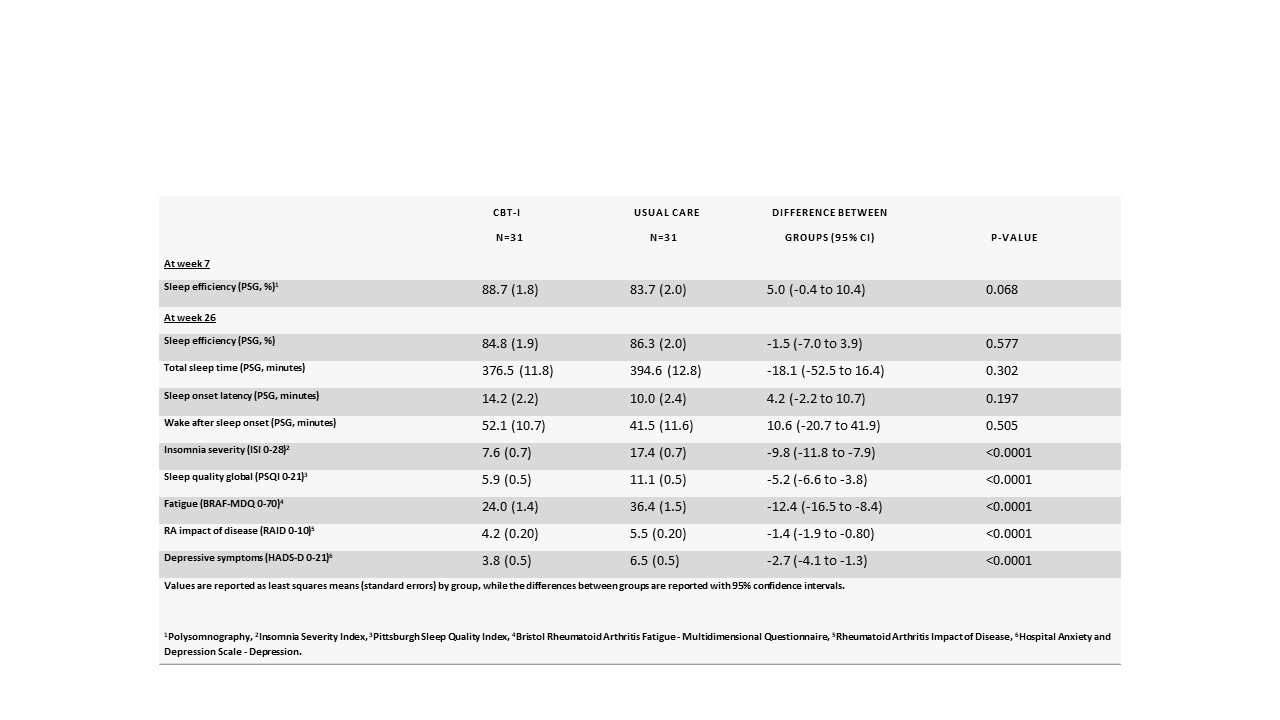 Table 1. Primary and key secondary outcomes at week 7 and week 26, and differences between treatment groups (based on the ITT population)
Table 1. Primary and key secondary outcomes at week 7 and week 26, and differences between treatment groups (based on the ITT population)
Disclosures: K. Latocha, None; K. Loeppenthin, None; M. Østergaard, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, UCB; p. jennum, None; M. Hetland, Sandoz, Novartis, Pfizer, Eli Lilly, Medac; H. Rogind, None; T. Lundbak, None; J. Midtgaard, None; R. Christensen, None; B. Esbensen, None.
Background/Purpose: Insomnia is highly prevalent in people with rheumatoid arthritis (RA) and may exacerbate symptoms and burdens, such as fatigue, depressive symptoms, and pain (1). Cognitive behavioral therapy for insomnia (CBT-I) has been shown to produce positive effects on sleep in other clinical populations (2,3). However, CBT-I has not previously been investigated in people with RA (1).
The primary objective was to compare the effect of nurse-led group-based CBT-I to usual care on sleep efficiency, measured by polysomnography (PSG) immediately after the intervention (i.e. seven weeks after baseline) in people with RA. Secondary objectives included comparing the longer-term effect of CBT-I on sleep and RA-related outcomes at 26 weeks' follow-up.
Methods: In a randomized controlled trial (ClinicalTrials.gov Identifier NCT03766100), using a parallel-group design, the experimental intervention was six weeks of CBT-I; the control comparator was usual care. CBT-I was delivered face-to-face by a CBT-I trained nurse. The primary analyses were based on the intention-to-treat (ITT) population; missing data were statistically handled using repeated-measures linear mixed-effects models adjusted for the level at baseline.
Results: The ITT population consisted of 62 participants (89% women), with an average age of 58 years (SD 10), DAS28-CRP of 3.4 (SD 1.0), Insomnia Severity Index (ISI) score of 18.9 (SD 4.4) and median Patient Global Assessment score of 55 (IQR 28;71).
When the primary outcome was measured by PSG at week seven, sleep efficiency was 88.7% in the CBT-I group, compared to 83.7% in the control group (difference: 5.0 [95% CI -0.4 to 10.4]; p=0.068) (See Table 1). Secondary outcomes measured by PSG had not improved at week 26 either.
However, for all secondary sleep and RA-related patient-reported outcomes, there were statistically highly significant differences between CBT-I and usual care e.g. Insomnia Severity Index (ISI, range 0-28, higher is worse): -9.8 [95% CI -11.8 to -7.9], RA impact of disease (RAID, range 0-10, higher is worse) -1.4 [95% CI-1.9 to -0.80] and Patient Global Assessment (range 0-100, higher is worse): -13.0 [95% CI -20.9 to -5.1] at 26 weeks' follow-up.
Conclusion: Nurse-led, group-based CBT-I for two hours per week for six weeks, did not improve objectively measured sleep efficiency or any other outcomes measured by PSG. However, CBT-I showed long-term improvement on patient-reported outcomes such as fatigue, impact of disease, depression, pain, and Patient Global Assessment – a finding that could have important clinical implications.
References
(1) PMID: 25620673
(2) PMID: 16804151
(3) PMID: 26434673
 Table 1. Primary and key secondary outcomes at week 7 and week 26, and differences between treatment groups (based on the ITT population)
Table 1. Primary and key secondary outcomes at week 7 and week 26, and differences between treatment groups (based on the ITT population)Disclosures: K. Latocha, None; K. Loeppenthin, None; M. Østergaard, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, UCB; p. jennum, None; M. Hetland, Sandoz, Novartis, Pfizer, Eli Lilly, Medac; H. Rogind, None; T. Lundbak, None; J. Midtgaard, None; R. Christensen, None; B. Esbensen, None.

