Back
Poster Session B
Infection-related rheumatic syndromes
Session: (0779–0806) Infection-related Rheumatic Disease Poster
0802: Is There Any Relationship Between Helicobacter Pylori and Rheumatoid Arthritis?
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- HW
Hui Wu, PhD
1. The Eighth Affiliated Hospital of Sun Yat-sen University
Shenzhen, Guangdong, China
Abstract Poster Presenter(s)
Hui Wu, Hanmei Yuan and Chao Wu, The Eighth Affiliated Hospital of Sun Yat-sen University, Shen zhen, China
Background/Purpose: Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by joint damage. Recently, several studies suggest that gut microbiota could promote RA progression. Helicobacter pylori (H. pylori), a spiral-shaped, flagellated, Gram-negative bacterium, is implicated in various autoimmune diseases. However, the relationship between H. pylori infection and RA remains poorly understood.
Methods: Twelve participants were selected for this study, including six Hp-infected patients and six uninfected individuals who served as control. RNA-seq data were obtained from the Gene Expression Omnibus (GEO) Database (accession: GSE27411). Differentially expressed mRNA was identified using the 'limma' R package. The adjusted P-value was analyzed to correct the false-positive results in GEO datasets. "Adjusted P < 0.05 and Log2(Fold Change) >1 or Log2(Fold Change)< −1" were set as the threshold for significant differential expression. To further confirm the underlying biological function of potential targets, we performed a functional enrichment analysis of data with the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases.
Results:
RNA-seq following H. pylori infection in gastric tissue identified 144 up-regulated genes while 52 down-regulated genes. Of particular concern was several numbers of CXC chemokine family, such as CXCL2, CXCL5, and CXCL8 (Fig.1A). Interestingly, up-regulated genes were significantly enriched in some signaling pathways, including rheumatoid arthritis, neutrophil migration, and immune response signaling pathways (Fig. 1B). Venn diagrams showed that eight genes were shared between rheumatoid arthritis and neutrophil migration (Fig.1C). These eight genes include Integrin beta chain-2 (ITGB2) which functions in cellular adhesion and cell surface signaling, but most of the function of the remaining genes is chemotaxis. Especially for CXCL5, it plays an essential role in regulating neutrophils.
Conclusion: H. pylori infection may be a contributing factor to RA pathogenesis by modulating neutrophils. Thus, additional study is needed to elucidate the underlying mechanism.
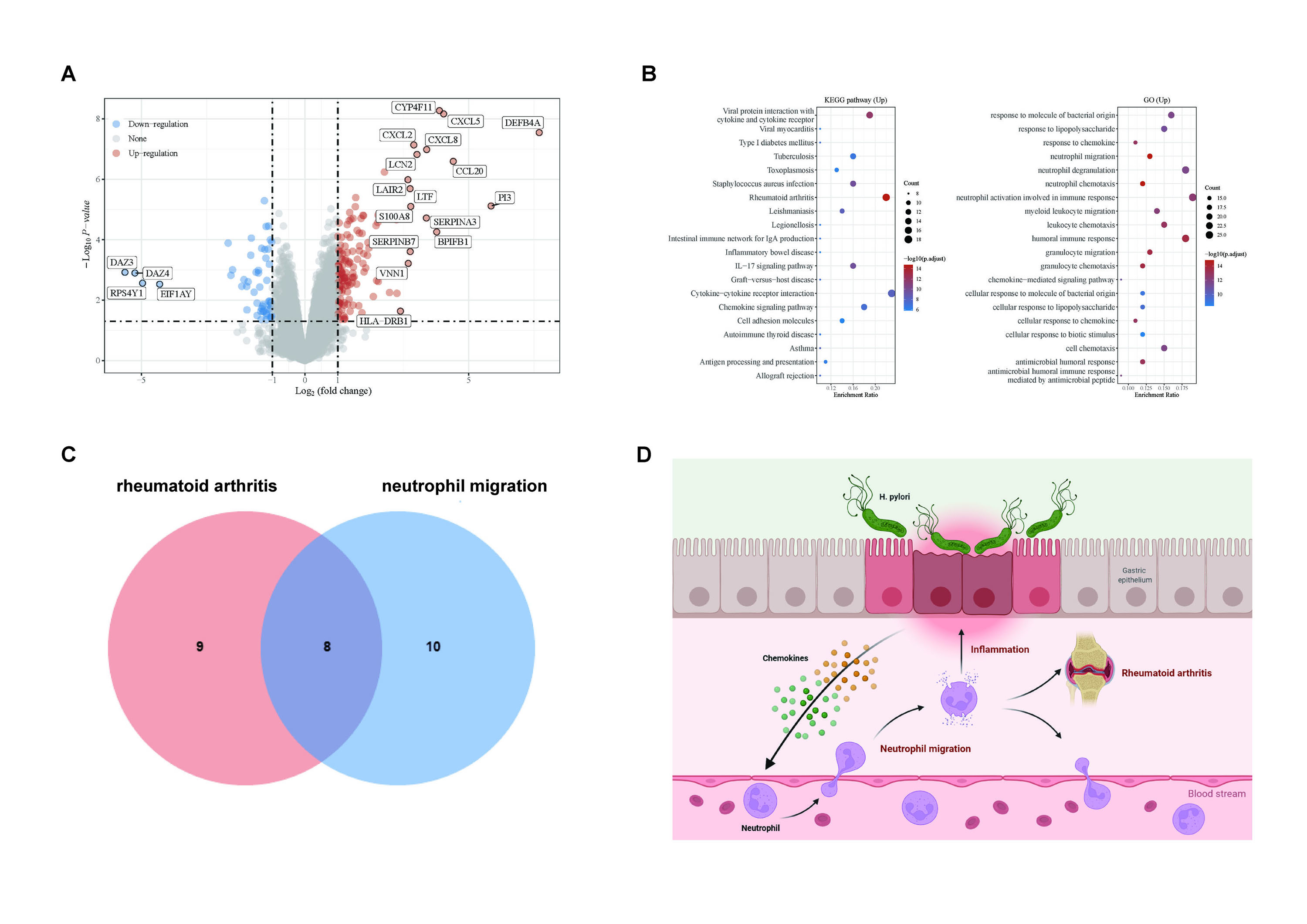 Figure1. Helicobacter pylori may play a crucial role in rheumatoid arthritis. (A) Volcano plot for differentially expressed genes. (B) KEGG and GO pathway analysis of up-regulated genes. (C) The Venn diagram displays the number of overlapped genes for rheumatoid arthritis and neutrophil migration. (D) A possible pathogenic mechanism of H. pylori.
Figure1. Helicobacter pylori may play a crucial role in rheumatoid arthritis. (A) Volcano plot for differentially expressed genes. (B) KEGG and GO pathway analysis of up-regulated genes. (C) The Venn diagram displays the number of overlapped genes for rheumatoid arthritis and neutrophil migration. (D) A possible pathogenic mechanism of H. pylori.
Disclosures: H. Wu, None; H. Yuan, None; C. Wu, None.
Background/Purpose: Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by joint damage. Recently, several studies suggest that gut microbiota could promote RA progression. Helicobacter pylori (H. pylori), a spiral-shaped, flagellated, Gram-negative bacterium, is implicated in various autoimmune diseases. However, the relationship between H. pylori infection and RA remains poorly understood.
Methods: Twelve participants were selected for this study, including six Hp-infected patients and six uninfected individuals who served as control. RNA-seq data were obtained from the Gene Expression Omnibus (GEO) Database (accession: GSE27411). Differentially expressed mRNA was identified using the 'limma' R package. The adjusted P-value was analyzed to correct the false-positive results in GEO datasets. "Adjusted P < 0.05 and Log2(Fold Change) >1 or Log2(Fold Change)< −1" were set as the threshold for significant differential expression. To further confirm the underlying biological function of potential targets, we performed a functional enrichment analysis of data with the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases.
Results:
RNA-seq following H. pylori infection in gastric tissue identified 144 up-regulated genes while 52 down-regulated genes. Of particular concern was several numbers of CXC chemokine family, such as CXCL2, CXCL5, and CXCL8 (Fig.1A). Interestingly, up-regulated genes were significantly enriched in some signaling pathways, including rheumatoid arthritis, neutrophil migration, and immune response signaling pathways (Fig. 1B). Venn diagrams showed that eight genes were shared between rheumatoid arthritis and neutrophil migration (Fig.1C). These eight genes include Integrin beta chain-2 (ITGB2) which functions in cellular adhesion and cell surface signaling, but most of the function of the remaining genes is chemotaxis. Especially for CXCL5, it plays an essential role in regulating neutrophils.
Conclusion: H. pylori infection may be a contributing factor to RA pathogenesis by modulating neutrophils. Thus, additional study is needed to elucidate the underlying mechanism.
 Figure1. Helicobacter pylori may play a crucial role in rheumatoid arthritis. (A) Volcano plot for differentially expressed genes. (B) KEGG and GO pathway analysis of up-regulated genes. (C) The Venn diagram displays the number of overlapped genes for rheumatoid arthritis and neutrophil migration. (D) A possible pathogenic mechanism of H. pylori.
Figure1. Helicobacter pylori may play a crucial role in rheumatoid arthritis. (A) Volcano plot for differentially expressed genes. (B) KEGG and GO pathway analysis of up-regulated genes. (C) The Venn diagram displays the number of overlapped genes for rheumatoid arthritis and neutrophil migration. (D) A possible pathogenic mechanism of H. pylori.Disclosures: H. Wu, None; H. Yuan, None; C. Wu, None.

