Back
Abstract Session
Session: Abstracts: Epidemiology and Public Health II: COVID, Infection and Pregnancy Outcomes (2191–2196)
2193: Invasive Fungal Diseases in Patients with Autoimmune Diseases: Prospective Data from a Nationwide French Registry
Monday, November 14, 2022
3:30 PM – 3:40 PM Eastern Time
Location: Room 204
- SG
Simon Galmiche, MD, MPH
Institut Pasteur
Paris, France
Presenting Author(s)
Simon Galmiche1, Benjamin Thoreau2, Stéphane Bretagne3, Alexandre Alanio3, André Paugam4, Valérie Bru5, Sophie Cassaing6, Jean-Pierre Gangneux7, Hélène Guégan7, Loïc Favennec8, Alida Minoza9, Florent Morio10, Julie Bonhomme11, Guillaume Desoubeaux12, Odile Eloy13, Lilia Hasseine14, Milène Sasso15, Laurence Millon16, Anne-Pauline Bellanger16, Philippe Poirier17, Maxime Moniot17, Taieb Chouaki18, Antoine Huguenin19, Frédéric Dalle20, Bernard Bouteille21, Muriel Nicolas22, Nicole Desbois-Nogard23, Marie-Elisabeth Bougnoux24, François Danion25, Vincent Poindron26, Antoine Néel27, Karine Boukris-Sitbon28, Fanny Lanternier29 and Benjamin Terrier30, 1Institut Pasteur, Paris, France, 2AP-HP, Université Paris Cité, INSERM U1016, CNR UMR 8104, Paris, France, 3Institut Pasteur, Université Paris Cité, Centre National de Référence Mycoses Invasives et Antifongiques, UMR 2000, AP-HP, Paris, France, 4Parasitologie – Mycologie, Université Paris Cité, Cochin Hospital, AP-HP, Paris, France, 5Institut de parasitologie et de pathologie tropicale, Hôpitaux universitaires de Strasbourg, Université de Strasbourg, Strasbourg, France, 6Parasitologie - Mycologie, Université de Toulouse, CHU de Toulouse, Toulouse, France, 7Université de Rennes, CHU, INSERM, Irset: Institut de Recherche en Santé, Environnement et Travail, UMR_S 1085, Rennes, France, 8French National Cryptosporidiosis Reference Center, Rouen University Hospital, Rouen, France, 9Département des agents infectieux, Service de Mycologie-Parasitologie, CHU de Poitiers, Poitiers, France, 10Parasitologe – Mycologie, CHU de Nantes, Nantes, France, 11Microbiologie, CHU de Caen, Caen, France, 12Parasitologie - Mycologie - Médecine tropicale, CHU de Tours, Tours, France, 13Microbiologie, Hôpital de Versailles, Le Chesnay, France, 14Parasitologie – Mycologie, hôpital de l'Archet, CHU Nice, Nice, France, 15Laboratoire de Parasitologie-Mycologie, CHU Nîmes, Université de Montpellier, CNRS, IRD, MiVEGEC, Nîmes, France, 16Laboratoire de Parasitologie – Mycologie, CHU Besançon, Besançon, France, 17Parasitologie – Mycologie, CHU de Clermont-Ferrand, Clermont-Ferrand, France, 18Mycologie – parasitologie, CHU d’Amiens, Amiens, France, 19Parasitologie – Mycologie, hôpital Maison-Blanche, CHU de Reims, Reims, France, 20Parasitologie – Mycologie, Plateforme de Biologie Hospitalo-Universitaire Gérard Mack, Dijon, France, 21Parasitologie – Mycologie, Centre de Biologie et de Recherche en Santé, CHU Dupuytren, Limoges, France, 22Mycologie – Parasitologie, CHU de Pointe-à-pitre/Abymes, Pointe-à-Pitre, France, 23Parasitologie – Mycologie, CHU de la Martinique, Fort-de-France, France, 24Institut Pasteur, Unité Biologie et Pathogénicité Fongiques, Département Mycologie, Laboratoire de Parasitologie – Mycologie, Service de Microbiologie, Necker-Enfants Malades University Hospital, AP-HP, Paris, France, 25Maladies infectieuses et tropicales, Hôpitaux universitaires de Strasbourg, Strasbourg, France, 26Immunologie clinique et médecine interne, Hôpitaux universitaires de Strasbourg, Strasbourg, France, 27CHU de Nantes, Nantes, France, 28Institut Pasteur, Université Paris Cité, CNRS, Mycologie Moléculaire, Centre National de Référence Mycoses Invasives et Antifongiques, UMR 2000, Paris, France, 29Infectious Diseases Unit, Necker-Enfants Malades University Hospital, AP-HP, Institut Pasteur, Université Paris Cité, Centre National de Référence Mycoses Invasives et Antifongiques, CNRS UMR 2000, Paris, France, 30National Referral Center for Rare Systemic Autoimmune Diseases, Cochin Hospital, Paris, France
Background/Purpose: Patients with autoimmune diseases (AIDs) display a risk of invasive fungal diseases (IFDs), because of the underlying disease or treatments used. Our objective was to describe the characteristics of IFDs in patients with AID and factors associated with mortality.
Methods: We analysed IFD cases included into a prospective multicenter registry conducted by the French national reference center for invasive mycoses and antifungals. We performed clustering after multiple correspondence analysis and a multivariable Cox model for factors associated with mortality.
Results: From 2012 to 2018, 552 individuals with IFD and AID were included, mainly with Pneumocystis jirovecii pneumonia (PCP) (n=229, 41.5%), fungemia (n=168, 30.4%) and invasive aspergillosis (n=84, 15.2%) (other IFD: n=58; multiple IFD: n=13). Rheumatoid arthritis (RA) and systemic vasculitis were the most frequent underlying AIDs in patients with PCP (respectively n=55 and n=42) and invasive aspergillosis (n=15 and n=14). Inflammatory bowel diseases (IBD) were predominant in fungemia (n=36). At IFD diagnosis, 346 (62.7%) patients received glucocorticoids (GCs) and 321 (58.2%) received immunosuppressive agents, mainly methotrexate (n=113), azathioprine (n=38) and cyclophosphamide (n=27). Mortality at 90 days was 34.5% (174/504), and was higher in invasive aspergillosis (31/81, 38.3%) and fungemia (72/148, 48.7%) compared to PCP (54/207, 26.1%) (p< 0.001) (figure 1). Fungemia (hazard ratio 2.4, 95% confidence interval 1.6-3.5, compared with PCP), advanced age (HR 2.1, 95% CI 1.3-3.2, for age ≥70 years compared with age < 55), cirrhosis (HR 3.7, 95% CI 2.1-6.4), and use of high-dose GCs ( > 0.3 mg/kg/day for > 1 month at IFD diagnosis) (HR 1.7, 95% CI 1.2-2.3) were independently associated with higher mortality. Clustering analysis identified three clusters: Cluster 1 consisted mainly of patients with PCP, IFD occurred 1 year after AID diagnosis in median, and almost all received high doses of GCs. Cluster 2 included mostly patients with RA, less frequently receiving GCs, and IFD occurred long after AID diagnosis (median 6 years). Cluster 3 included almost exclusively fungemia, occurring long after AID diagnosis (median 8 years). Cluster 2 (24.4%) displayed a lower mortality than the other two (cluster 1: 39.5%; cluster 3: 40.5%; p = 0.002). PCP (n=239) mostly occurred early after AID diagnosis (median 2 years, interquartile range 0-11) except for cases with RA (9 years, IQR 3-18). CD4+ T cells were measured within 3 months of PCP diagnosis for 78 patients (32.6%): median level was 472.5/mm3 (IQR 198-858), 49.6% (IQR 36.8-65.3) and CD4/CD8 ratio 2.2 (IQR 1.3-4.3). Among cases of fungemia, patients with IBD had an improved survival (HR 0.19, 95% CI 0.07-0.52).
Conclusion: IFDs are associated with a high mortality in patients with AID. PCP is the main IFD in this population and occurs primarily in patients with RA. Fungemia (apart for patients with IBD), high-dose GCs, cirrhosis and older age are associated with poor survival. PCP occurs usually early after AID onset except in cases of RA. CD4 T cell lymphopenia, mostly absent in cases of PCP, poorly predicts the risk of PCP in patients with AID.
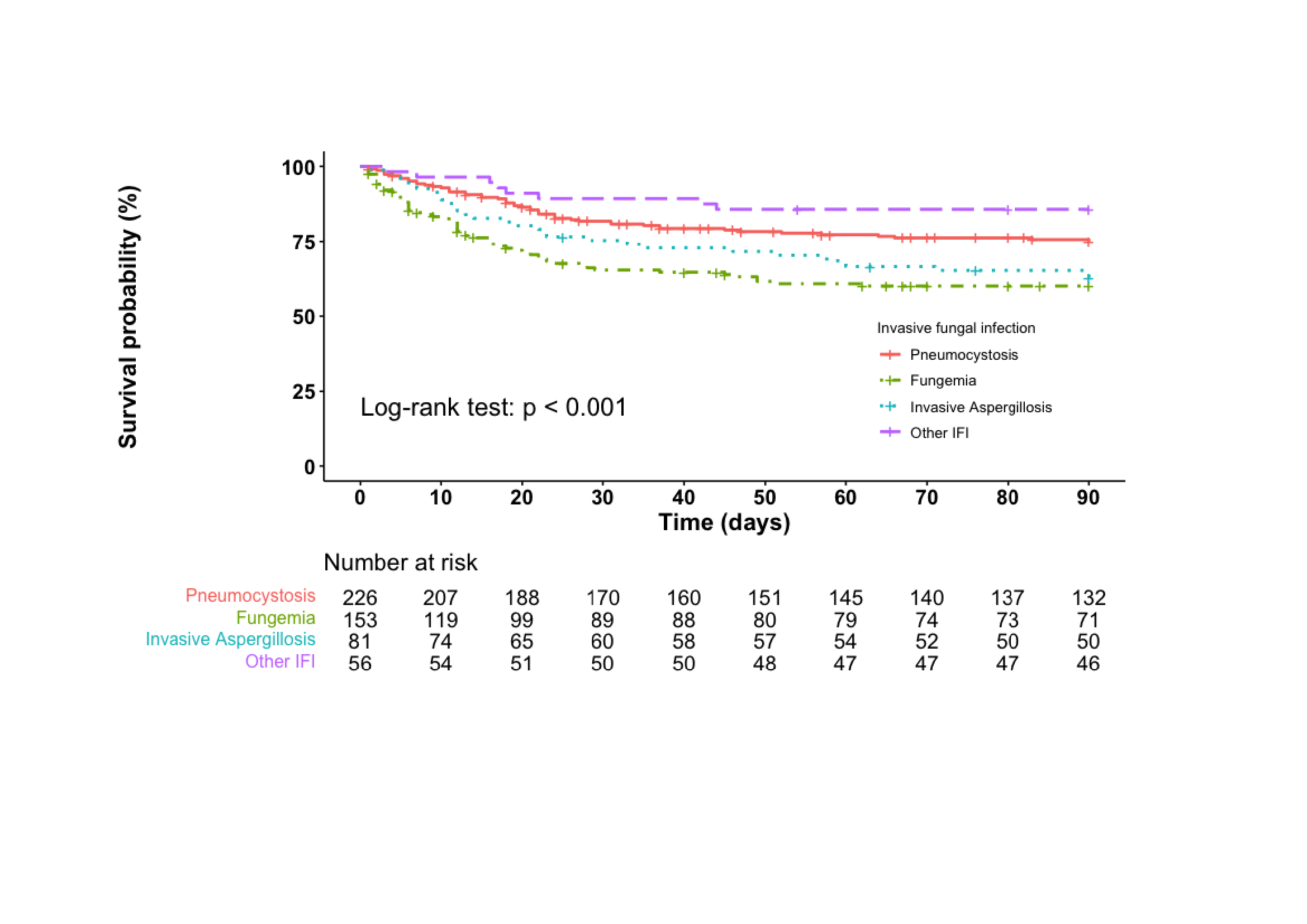 Figure 1: Kaplan-Meier analysis of survival in the 90 days following diagnosis of the invasive fungal disease (IFD) according to the type of IFD
Figure 1: Kaplan-Meier analysis of survival in the 90 days following diagnosis of the invasive fungal disease (IFD) according to the type of IFD
Legend: patients with multiple IFDs were not included in the analysis; 23 participants (including 21 dead and 2 lost to follow-up) were censored before date of diagnosis and were not included in the analysis
Disclosures: S. Galmiche, None; B. Thoreau, None; S. Bretagne, None; A. Alanio, None; A. Paugam, None; V. Bru, None; S. Cassaing, None; J. Gangneux, None; H. Guégan, None; L. Favennec, None; A. Minoza, None; F. Morio, None; J. Bonhomme, None; G. Desoubeaux, None; O. Eloy, None; L. Hasseine, None; M. Sasso, None; L. Millon, None; A. Bellanger, None; P. Poirier, None; M. Moniot, None; T. Chouaki, None; A. Huguenin, None; F. Dalle, None; B. Bouteille, None; M. Nicolas, None; N. Desbois-Nogard, None; M. Bougnoux, None; F. Danion, None; V. Poindron, None; A. Néel, None; K. Boukris-Sitbon, None; F. Lanternier, None; B. Terrier, AstraZeneca, GlaxoSmithKline, Bristol-Myers Squibb(BMS), Eli Lilly, LFB, Boehinger Ingelheim, Vifor Pharma, Pfizer, Roche.
Background/Purpose: Patients with autoimmune diseases (AIDs) display a risk of invasive fungal diseases (IFDs), because of the underlying disease or treatments used. Our objective was to describe the characteristics of IFDs in patients with AID and factors associated with mortality.
Methods: We analysed IFD cases included into a prospective multicenter registry conducted by the French national reference center for invasive mycoses and antifungals. We performed clustering after multiple correspondence analysis and a multivariable Cox model for factors associated with mortality.
Results: From 2012 to 2018, 552 individuals with IFD and AID were included, mainly with Pneumocystis jirovecii pneumonia (PCP) (n=229, 41.5%), fungemia (n=168, 30.4%) and invasive aspergillosis (n=84, 15.2%) (other IFD: n=58; multiple IFD: n=13). Rheumatoid arthritis (RA) and systemic vasculitis were the most frequent underlying AIDs in patients with PCP (respectively n=55 and n=42) and invasive aspergillosis (n=15 and n=14). Inflammatory bowel diseases (IBD) were predominant in fungemia (n=36). At IFD diagnosis, 346 (62.7%) patients received glucocorticoids (GCs) and 321 (58.2%) received immunosuppressive agents, mainly methotrexate (n=113), azathioprine (n=38) and cyclophosphamide (n=27). Mortality at 90 days was 34.5% (174/504), and was higher in invasive aspergillosis (31/81, 38.3%) and fungemia (72/148, 48.7%) compared to PCP (54/207, 26.1%) (p< 0.001) (figure 1). Fungemia (hazard ratio 2.4, 95% confidence interval 1.6-3.5, compared with PCP), advanced age (HR 2.1, 95% CI 1.3-3.2, for age ≥70 years compared with age < 55), cirrhosis (HR 3.7, 95% CI 2.1-6.4), and use of high-dose GCs ( > 0.3 mg/kg/day for > 1 month at IFD diagnosis) (HR 1.7, 95% CI 1.2-2.3) were independently associated with higher mortality. Clustering analysis identified three clusters: Cluster 1 consisted mainly of patients with PCP, IFD occurred 1 year after AID diagnosis in median, and almost all received high doses of GCs. Cluster 2 included mostly patients with RA, less frequently receiving GCs, and IFD occurred long after AID diagnosis (median 6 years). Cluster 3 included almost exclusively fungemia, occurring long after AID diagnosis (median 8 years). Cluster 2 (24.4%) displayed a lower mortality than the other two (cluster 1: 39.5%; cluster 3: 40.5%; p = 0.002). PCP (n=239) mostly occurred early after AID diagnosis (median 2 years, interquartile range 0-11) except for cases with RA (9 years, IQR 3-18). CD4+ T cells were measured within 3 months of PCP diagnosis for 78 patients (32.6%): median level was 472.5/mm3 (IQR 198-858), 49.6% (IQR 36.8-65.3) and CD4/CD8 ratio 2.2 (IQR 1.3-4.3). Among cases of fungemia, patients with IBD had an improved survival (HR 0.19, 95% CI 0.07-0.52).
Conclusion: IFDs are associated with a high mortality in patients with AID. PCP is the main IFD in this population and occurs primarily in patients with RA. Fungemia (apart for patients with IBD), high-dose GCs, cirrhosis and older age are associated with poor survival. PCP occurs usually early after AID onset except in cases of RA. CD4 T cell lymphopenia, mostly absent in cases of PCP, poorly predicts the risk of PCP in patients with AID.
 Figure 1: Kaplan-Meier analysis of survival in the 90 days following diagnosis of the invasive fungal disease (IFD) according to the type of IFD
Figure 1: Kaplan-Meier analysis of survival in the 90 days following diagnosis of the invasive fungal disease (IFD) according to the type of IFDLegend: patients with multiple IFDs were not included in the analysis; 23 participants (including 21 dead and 2 lost to follow-up) were censored before date of diagnosis and were not included in the analysis
Disclosures: S. Galmiche, None; B. Thoreau, None; S. Bretagne, None; A. Alanio, None; A. Paugam, None; V. Bru, None; S. Cassaing, None; J. Gangneux, None; H. Guégan, None; L. Favennec, None; A. Minoza, None; F. Morio, None; J. Bonhomme, None; G. Desoubeaux, None; O. Eloy, None; L. Hasseine, None; M. Sasso, None; L. Millon, None; A. Bellanger, None; P. Poirier, None; M. Moniot, None; T. Chouaki, None; A. Huguenin, None; F. Dalle, None; B. Bouteille, None; M. Nicolas, None; N. Desbois-Nogard, None; M. Bougnoux, None; F. Danion, None; V. Poindron, None; A. Néel, None; K. Boukris-Sitbon, None; F. Lanternier, None; B. Terrier, AstraZeneca, GlaxoSmithKline, Bristol-Myers Squibb(BMS), Eli Lilly, LFB, Boehinger Ingelheim, Vifor Pharma, Pfizer, Roche.

