Back
Poster Session B
Juvenile idiopathic arthritis (JIA) and pediatric joint disorders
Session: (0850–0880) Pediatric Rheumatology – Clinical Poster I: JIA
0870: Knee Acoustic Emissions as a Noninvasive Biomarker of Articular Health in Juvenile Idiopathic Arthritis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- SG
Sevda Gharehbaghi, PhD
Georgia Institute of Technology
Santa Clara, CA, United States
Abstract Poster Presenter(s)
Sevda Gharehbaghi1, Emily Moise1, Priya Brito2, Lori Ponder3, Omer Inan1 and Sampath Prahalad4, 1Georgia Institute of Technology, Atlanta, GA, 2Emory University, Atlanta, GA, 3Children's Healthcare of Atlanta, Atlanta, GA, 4Emory + Children's Pediatric Institute, Atlanta, GA
Background/Purpose: Juvenile idiopathic arthritis (JIA) refers to all forms of autoimmune arthritis in children with undetermined etiology, and thus it is the most prevalent chronic arthritis in children. Hallmark symptoms of JIA include swelling, warmth, pain, and stiffness in the joints, particularly in the knee, the most commonly affected joint. For clinical trials, a joint with either swelling alone, and/or tenderness, and limited motion is considered active. The chronic inflammation of the joint causes damage to the smooth and slippery underlying cartilage and even bone if left untreated. Current diagnostic methods involve insensitive and subjective physician examinations, general symptom-based patient surveys, and non-specific laboratory tests. Crepitus emitted from inter-joint articulating surfaces, aka joint acoustic emissions (JAEs), can be recorded with highly sensitive contact microphones and used as a non-invasive biomarker of articular health in children with JIA, allowing differentiation between healthy and arthritic joints and particularly active and inactive involvement.
Methods: Knee JAEs were acquired from 117 participants while subjects were asked to perform 10 repetitions of seated knee flexion/extensions. Study participants included 30 healthy controls and 87 subjects with JIA where 43 knees had active JIA involvement and 131 knees had inactive JIA involvement. These JAE signals were filtered, denoised, and then divided into short-time frames to extract 273 time-frequency audio features, from which 60 top Principal Components (PC), explaining 95% of the data variance, were obtained as the training data for an Extreme Gradient Boosting (XGBoost) machine learning classification model. Knees with active JIA involvement were assumed to have more certain labels compared to inactive cases. Therefore, the classifier was trained on all knees with active JIA and 80% of the healthy controls and then tested on the knees with inactive JIA and the rest of the healthy controls. The classifier predicted a knee health score for each leg as the probability of having JIA.
Results: The classifier was validated on the training data through a leave-one-leg-out cross-validation with 81% accuracy. Then, it was tested on the knees with inactive JIA involvement and remaining healthy knees, resulting in 87% accuracy. The validation and test confusion matrices are shown in Fig. 1 (a–b). The health score distribution of knees with active involvement was significantly higher than those with inactive involvement (Fig. 1 (d)). Furthermore, from 27 subjects with unilateral knee involvement, 70% of the active knees were correctly assigned to a higher JIA probability compared to inactive knees.
Conclusion: This study obtains knee JAEs from a larger population compared to our previous study and it demonstrates a more generalizable model for knee health assessment in children with JIA. Therefore, it supports the use of JAEs as a novel non-invasive digital biomarker for Articular Health assessment. Furthermore, knee health scores can differentiate between active and inactive JIA and can be used to better personalize the treatment.
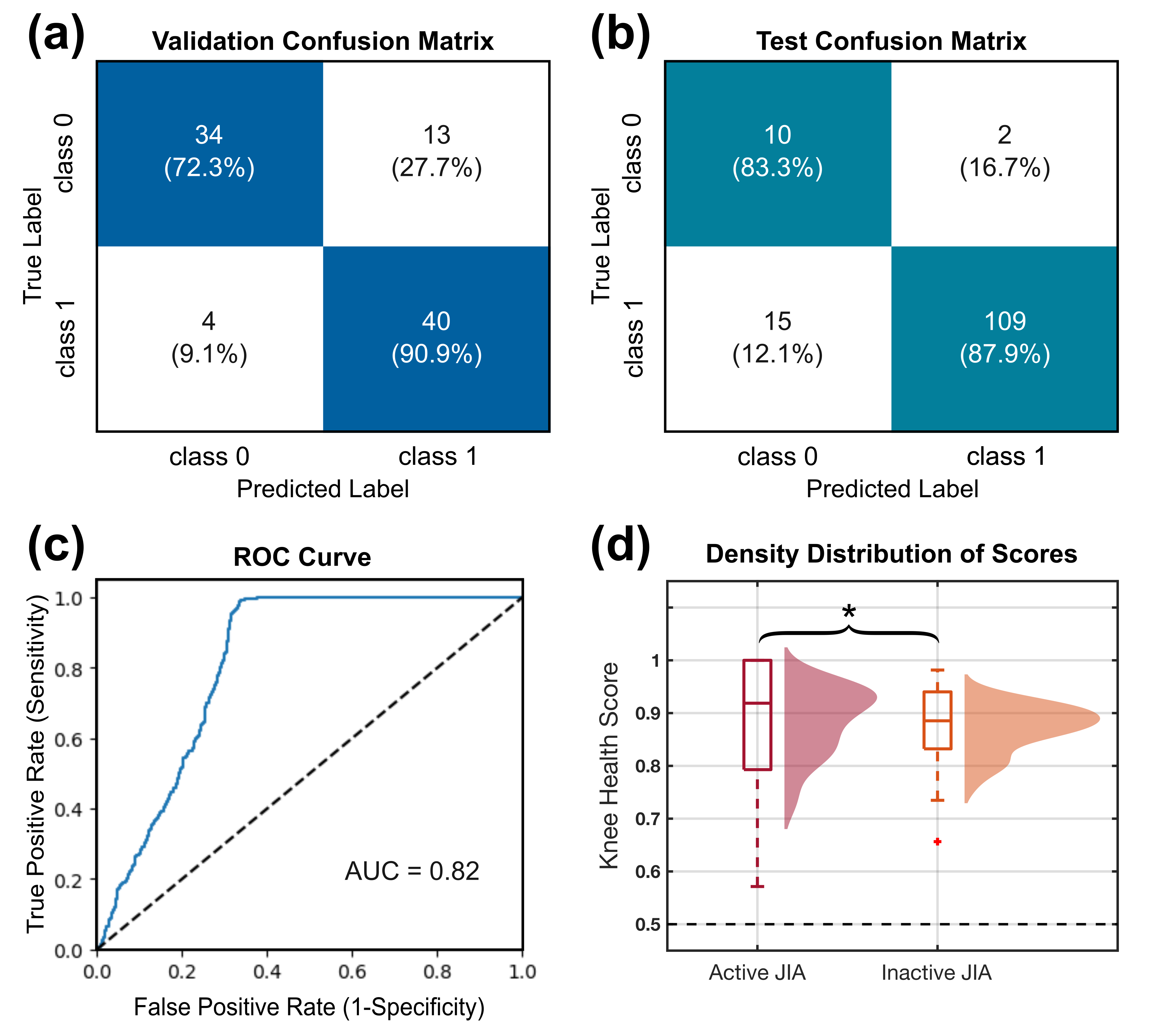 Results of classification, including the confusion matrix of leave-one-leg-out validation on the training data (a) and testing data (b), receiver operating characteristic (ROC) curve of the classifier (c), and the density distribution of the scores from active and inactive legs of subjects with asymmetric JIA involvement.
Results of classification, including the confusion matrix of leave-one-leg-out validation on the training data (a) and testing data (b), receiver operating characteristic (ROC) curve of the classifier (c), and the density distribution of the scores from active and inactive legs of subjects with asymmetric JIA involvement.
Disclosures: S. Gharehbaghi, None; E. Moise, None; P. Brito, None; L. Ponder, None; O. Inan, Arthroba; S. Prahalad, Novartis.
Background/Purpose: Juvenile idiopathic arthritis (JIA) refers to all forms of autoimmune arthritis in children with undetermined etiology, and thus it is the most prevalent chronic arthritis in children. Hallmark symptoms of JIA include swelling, warmth, pain, and stiffness in the joints, particularly in the knee, the most commonly affected joint. For clinical trials, a joint with either swelling alone, and/or tenderness, and limited motion is considered active. The chronic inflammation of the joint causes damage to the smooth and slippery underlying cartilage and even bone if left untreated. Current diagnostic methods involve insensitive and subjective physician examinations, general symptom-based patient surveys, and non-specific laboratory tests. Crepitus emitted from inter-joint articulating surfaces, aka joint acoustic emissions (JAEs), can be recorded with highly sensitive contact microphones and used as a non-invasive biomarker of articular health in children with JIA, allowing differentiation between healthy and arthritic joints and particularly active and inactive involvement.
Methods: Knee JAEs were acquired from 117 participants while subjects were asked to perform 10 repetitions of seated knee flexion/extensions. Study participants included 30 healthy controls and 87 subjects with JIA where 43 knees had active JIA involvement and 131 knees had inactive JIA involvement. These JAE signals were filtered, denoised, and then divided into short-time frames to extract 273 time-frequency audio features, from which 60 top Principal Components (PC), explaining 95% of the data variance, were obtained as the training data for an Extreme Gradient Boosting (XGBoost) machine learning classification model. Knees with active JIA involvement were assumed to have more certain labels compared to inactive cases. Therefore, the classifier was trained on all knees with active JIA and 80% of the healthy controls and then tested on the knees with inactive JIA and the rest of the healthy controls. The classifier predicted a knee health score for each leg as the probability of having JIA.
Results: The classifier was validated on the training data through a leave-one-leg-out cross-validation with 81% accuracy. Then, it was tested on the knees with inactive JIA involvement and remaining healthy knees, resulting in 87% accuracy. The validation and test confusion matrices are shown in Fig. 1 (a–b). The health score distribution of knees with active involvement was significantly higher than those with inactive involvement (Fig. 1 (d)). Furthermore, from 27 subjects with unilateral knee involvement, 70% of the active knees were correctly assigned to a higher JIA probability compared to inactive knees.
Conclusion: This study obtains knee JAEs from a larger population compared to our previous study and it demonstrates a more generalizable model for knee health assessment in children with JIA. Therefore, it supports the use of JAEs as a novel non-invasive digital biomarker for Articular Health assessment. Furthermore, knee health scores can differentiate between active and inactive JIA and can be used to better personalize the treatment.
 Results of classification, including the confusion matrix of leave-one-leg-out validation on the training data (a) and testing data (b), receiver operating characteristic (ROC) curve of the classifier (c), and the density distribution of the scores from active and inactive legs of subjects with asymmetric JIA involvement.
Results of classification, including the confusion matrix of leave-one-leg-out validation on the training data (a) and testing data (b), receiver operating characteristic (ROC) curve of the classifier (c), and the density distribution of the scores from active and inactive legs of subjects with asymmetric JIA involvement.Disclosures: S. Gharehbaghi, None; E. Moise, None; P. Brito, None; L. Ponder, None; O. Inan, Arthroba; S. Prahalad, Novartis.

