Back
Poster Session B
Infection-related rheumatic syndromes
Session: (0779–0806) Infection-related Rheumatic Disease Poster
0782: Low Circulating B Cells Correlate with Unresponsiveness to COVID-19 Vaccination Among Patients Treated with Rituximab
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- CH
Christopher Halbur, BS
University of Iowa
Iowa City, IA, United States
Abstract Poster Presenter(s)
Christopher Halbur1, Erin Sternhagen2, Mohamad Dave Dimachkie2 and Petar Lenert2, 1University of Iowa Carver College of Medicine, Iowa City, IA, 2University of Iowa Hospitals and Clinics, Iowa City, IA
Background/Purpose: Rituximab impairs humoral immunity by depleting CD20+ B cells. Previous studies have demonstrated that patients on rituximab have decreased immune response to COVID-19 vaccination. However, prior studies have not fully delineated the effect of patient characteristics, treatment protocol, timing of vaccination, and B cell levels on antibody response. This study aims to assess how patient characteristics impact COVID-19 seroconversion in patients treated with rituximab.
Methods: This was a single-center retrospective cohort study including 77 patients with rheumatoid arthritis (RA), ANCA-associated vasculitis (AAV), and SLE/mixed connective tissue disease who obtained COVID-19 antibody testing at clinic visits from May to December 2021. 48 (62%) of these patients received rituximab. The Roche Elecsys anti-SARS-CoV-2 (S) immunoassay was used to determine the presence of antibodies. Seroconversion was defined by antibodies ≥ 0.8 U/mL. Characteristics of rituximab-receiving patients that seroconverted were compared to those that did not seroconvert, using Fisher's exact test for categorical variables and a student's t-test for continuous variables. A multivariable logistic regression was used to adjust for selected covariates.
Results: Fewer patients on rituximab seroconverted (46%) compared to those treated with non-B cell targeting agents (86%) (p< 0.001). Among patients on rituximab, factors associated with seroconversion included longer duration between rituximab and 2nd vaccine (p=0.03), presence of anti-nucleocapsid antibodies (p< 0.01), more CD19+ lymphocytes (p=0.02), and greater serum IgG levels (p< 0.001) (Table 1). Patients with serum IgG ≤700 mg/dL had lower odds of seroconversion than patients with IgG >700 mg/dL (OR=0.19; 95% CI: 0.05-0.70). Patients with < 1% CD19+ lymphocytes were less likely to have anti-S antibodies than patients with higher B cell levels (OR=0.15; CI: 0.03-0.66). < 1% CD19 remained associated with lower odds of seroconversion after adjustment for covariates (aOR=0.13; 95% CI: 0.02-0.82) (Figure 1). Quantitative anti-S titer was positively correlated with serum IgG (r=0.31), %CD19+ lymphocytes (r=0.37), and time between rituximab and vaccine (r=0.49) (Figure 2).
There was no difference in seroconversion between patients with AAV and RA (p=0.76). There was no difference in seroconversion based on patient age, sex, vaccine received, number of vaccine doses, months between vaccine and antibody test, or %CD3+ lymphocytes. Six patients (13%) developed subsequent COVID-19 infection as of May 2022; none required hospitalization. Eight patients that did not initially have antibodies were re-tested after receiving a booster vaccine within 6 months. Only one of these patients seroconverted following a booster dose.
Conclusion: Despite different treatment protocols, patients with rheumatoid arthritis and ANCA-associated vasculitis on rituximab had similar rates of antibody response to COVID-19 vaccination. Low levels of circulating B cells and serum IgG levels were associated with decreased antibody response. This study adds to a growing body of evidence that treatment with rituximab is associated with suppressed response to COVID-19 vaccination.
.jpg) Table 1. Characteristics of rheumatology patients receiving rituximab, stratified by seroconversion. Odds ratios calculated where applicable.
Table 1. Characteristics of rheumatology patients receiving rituximab, stratified by seroconversion. Odds ratios calculated where applicable.
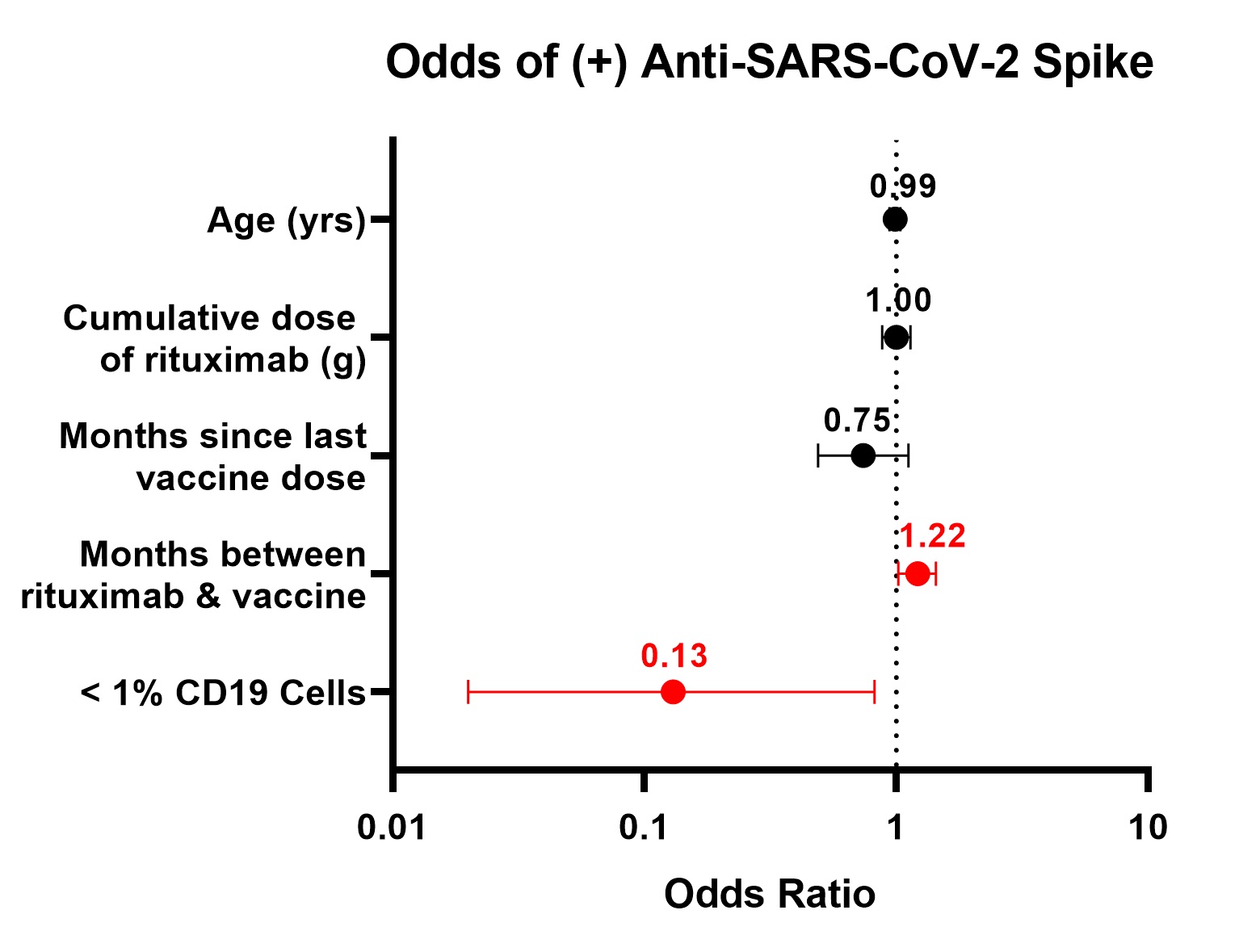 Figure 1. Adjusted odds of seroconversion to SARS-CoV-2 spike protein, multivariable logistic regression
Figure 1. Adjusted odds of seroconversion to SARS-CoV-2 spike protein, multivariable logistic regression
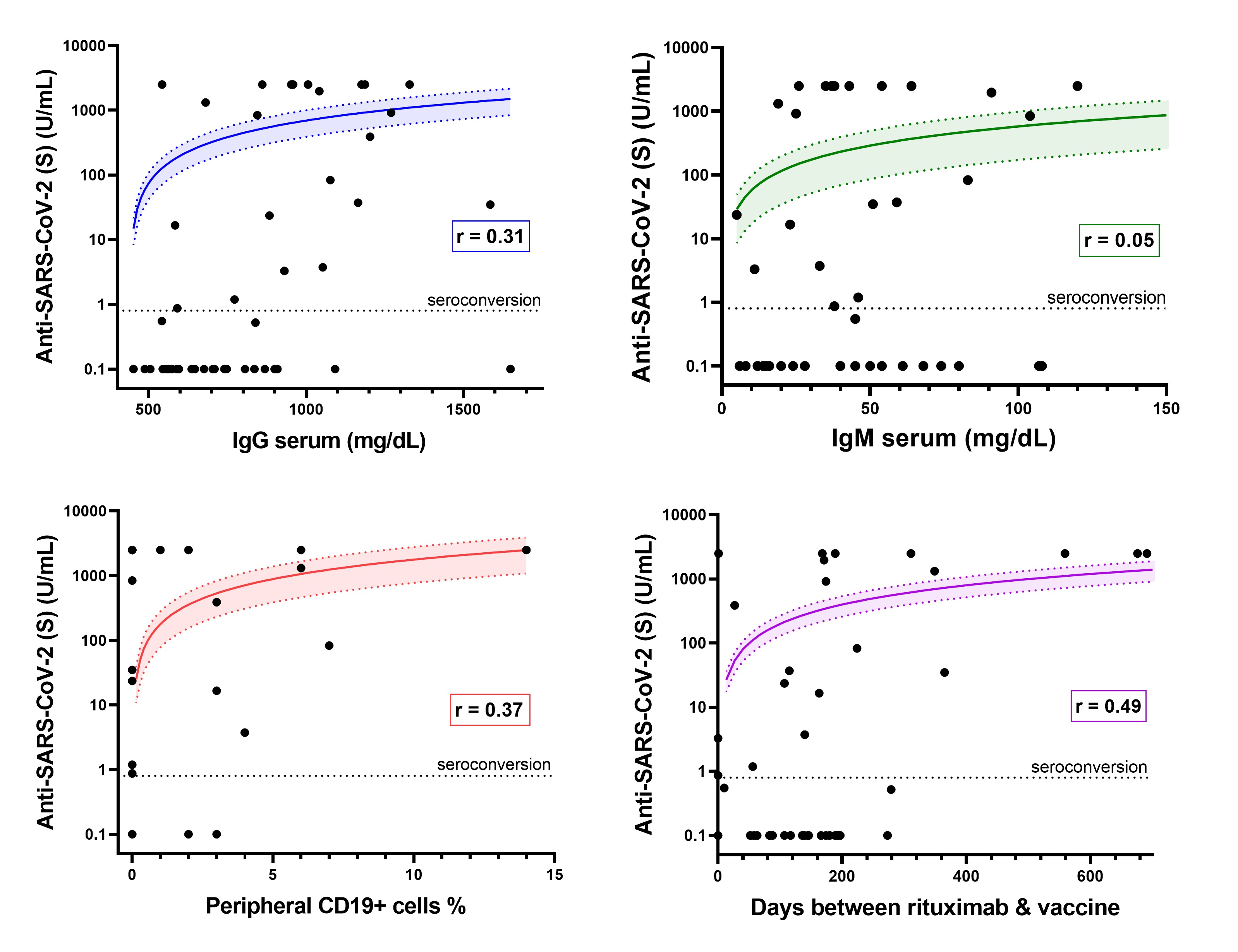 Figure 2. Association between anti-SARS-CoV-2 titer (U/mL) and selected variables. Lines represent linear regression fit, which appears curved on logarithmic axes. Shaded area represents 95% confidence interval of regression fit. Seroconversion defined by anti-S ≥0.8 U/mL.
Figure 2. Association between anti-SARS-CoV-2 titer (U/mL) and selected variables. Lines represent linear regression fit, which appears curved on logarithmic axes. Shaded area represents 95% confidence interval of regression fit. Seroconversion defined by anti-S ≥0.8 U/mL.
Disclosures: C. Halbur, None; E. Sternhagen, None; M. Dimachkie, None; P. Lenert, None.
Background/Purpose: Rituximab impairs humoral immunity by depleting CD20+ B cells. Previous studies have demonstrated that patients on rituximab have decreased immune response to COVID-19 vaccination. However, prior studies have not fully delineated the effect of patient characteristics, treatment protocol, timing of vaccination, and B cell levels on antibody response. This study aims to assess how patient characteristics impact COVID-19 seroconversion in patients treated with rituximab.
Methods: This was a single-center retrospective cohort study including 77 patients with rheumatoid arthritis (RA), ANCA-associated vasculitis (AAV), and SLE/mixed connective tissue disease who obtained COVID-19 antibody testing at clinic visits from May to December 2021. 48 (62%) of these patients received rituximab. The Roche Elecsys anti-SARS-CoV-2 (S) immunoassay was used to determine the presence of antibodies. Seroconversion was defined by antibodies ≥ 0.8 U/mL. Characteristics of rituximab-receiving patients that seroconverted were compared to those that did not seroconvert, using Fisher's exact test for categorical variables and a student's t-test for continuous variables. A multivariable logistic regression was used to adjust for selected covariates.
Results: Fewer patients on rituximab seroconverted (46%) compared to those treated with non-B cell targeting agents (86%) (p< 0.001). Among patients on rituximab, factors associated with seroconversion included longer duration between rituximab and 2nd vaccine (p=0.03), presence of anti-nucleocapsid antibodies (p< 0.01), more CD19+ lymphocytes (p=0.02), and greater serum IgG levels (p< 0.001) (Table 1). Patients with serum IgG ≤700 mg/dL had lower odds of seroconversion than patients with IgG >700 mg/dL (OR=0.19; 95% CI: 0.05-0.70). Patients with < 1% CD19+ lymphocytes were less likely to have anti-S antibodies than patients with higher B cell levels (OR=0.15; CI: 0.03-0.66). < 1% CD19 remained associated with lower odds of seroconversion after adjustment for covariates (aOR=0.13; 95% CI: 0.02-0.82) (Figure 1). Quantitative anti-S titer was positively correlated with serum IgG (r=0.31), %CD19+ lymphocytes (r=0.37), and time between rituximab and vaccine (r=0.49) (Figure 2).
There was no difference in seroconversion between patients with AAV and RA (p=0.76). There was no difference in seroconversion based on patient age, sex, vaccine received, number of vaccine doses, months between vaccine and antibody test, or %CD3+ lymphocytes. Six patients (13%) developed subsequent COVID-19 infection as of May 2022; none required hospitalization. Eight patients that did not initially have antibodies were re-tested after receiving a booster vaccine within 6 months. Only one of these patients seroconverted following a booster dose.
Conclusion: Despite different treatment protocols, patients with rheumatoid arthritis and ANCA-associated vasculitis on rituximab had similar rates of antibody response to COVID-19 vaccination. Low levels of circulating B cells and serum IgG levels were associated with decreased antibody response. This study adds to a growing body of evidence that treatment with rituximab is associated with suppressed response to COVID-19 vaccination.
.jpg) Table 1. Characteristics of rheumatology patients receiving rituximab, stratified by seroconversion. Odds ratios calculated where applicable.
Table 1. Characteristics of rheumatology patients receiving rituximab, stratified by seroconversion. Odds ratios calculated where applicable.  Figure 1. Adjusted odds of seroconversion to SARS-CoV-2 spike protein, multivariable logistic regression
Figure 1. Adjusted odds of seroconversion to SARS-CoV-2 spike protein, multivariable logistic regression  Figure 2. Association between anti-SARS-CoV-2 titer (U/mL) and selected variables. Lines represent linear regression fit, which appears curved on logarithmic axes. Shaded area represents 95% confidence interval of regression fit. Seroconversion defined by anti-S ≥0.8 U/mL.
Figure 2. Association between anti-SARS-CoV-2 titer (U/mL) and selected variables. Lines represent linear regression fit, which appears curved on logarithmic axes. Shaded area represents 95% confidence interval of regression fit. Seroconversion defined by anti-S ≥0.8 U/mL. Disclosures: C. Halbur, None; E. Sternhagen, None; M. Dimachkie, None; P. Lenert, None.

