Back
Abstract Session
Session: Abstracts: Epidemiology and Public Health II: COVID, Infection and Pregnancy Outcomes (2191–2196)
2194: Is Vaccination Against Covid-19 Associated with Autoimmune Rheumatic Disease Flare? Self-controlled Case Series Analysis Using Data from the Clinical Practice Research Datalink (Aurum)
Monday, November 14, 2022
3:45 PM – 3:55 PM Eastern Time
Location: Room 204
- GN
Georgina Nakafero, PhD
University of Nottingham
Nottingham, United Kingdom
Presenting Author(s)
Georgina Nakafero1, Matthew J. Grainge1, Tim Card1, Christian Mallen2, Jonathan S. Nguyen van Tam1, Hywel C. Williams1 and Abhishek Abhishek1, 1University of Nottingham, Nottingham, United Kingdom, 2Keele University, Keele, United Kingdom
Background/Purpose: It is unclear whether autoimmune rheumatic disease (AIRD) flares are associated with the recent prior Covid-19 vaccination, and whether the association was influenced by vaccine modality and prior Covid-19 infection. The objectives of this study were to investigate the association between vaccination against Covid-19 and AIRD flare and whether the association varied for sequential vaccinations, according to types of AIRDs, prior Covid-19, and between mRNA and vectored DNA vaccines.
Methods: We employed self-controlled case-series analysis, a method that is extensively used in vaccine safety studies because it takes between person confounding into account. Patients with AIRDs vaccinated against Covid-19 who consulted for disease flare between 01/12/2020 and 31/12/2021 were ascertained from the Clinical Practice Research Datalink (Aurum). AIRD flare was defined as consultation for AIRD with corticosteroid prescription on the same day or the next day. Vaccination was defined using date of vaccination and product code. The observation period was partitioned into vaccine-exposed (21-days after vaccination), pre-vaccination (7-days before vaccination), and remaining vaccine-unexposed periods (Figure 1). Participants contributed data with multiple vaccinations and outcomes. Season adjusted incidence rate ratios (aIRR) and 95% confidence intervals (CI) were calculated to compare the frequency of AIRD flare in different periods.
Results: Data for 3554 AIRD cases, 72% female, mean age 65 years, and 68.3% with rheumatoid arthritis were included. Covid-19 vaccination was associated with significantly fewer AIRD flares in the 21-day vaccine-exposed period when all vaccinations were considered (aIRR(95%CI) 0.89(0.80-0.98)). Using dose-stratified analyses there was a statistically significant negative association in 21-days after first Covid-19 vaccination but no association after the second or third Covid-19 vaccinations (aIRR(95%CI) 0.76(0.66-0.89), 0.94(0.79-1.11) and 1.01(0.85-1.20) respectively) (Table 1). On AIRD type stratified analyses, vaccination was not associated with disease flares. Vaccination without or after SARS-CoV-2 infection, and with vectored DNA or mRNA vaccines associated with comparable reduced risk of AIRD flares in the vaccine-exposed period after first Covid-19 vaccination (Table 2).
Conclusion: Vaccination against Covid-19 was not associated with increased AIRD flare, and vaccination with or without prior Covid-19, and with either mRNA or vectored DNA vaccines were not associated with AIRD flares. These data should address the apprehension of disease specific adverse effects from Covid-19 vaccination, an important reason for vaccine hesitancy in AIRDs, that may become even more significant as a barrier against vaccination as the perceived benefit from booster vaccination reduces.
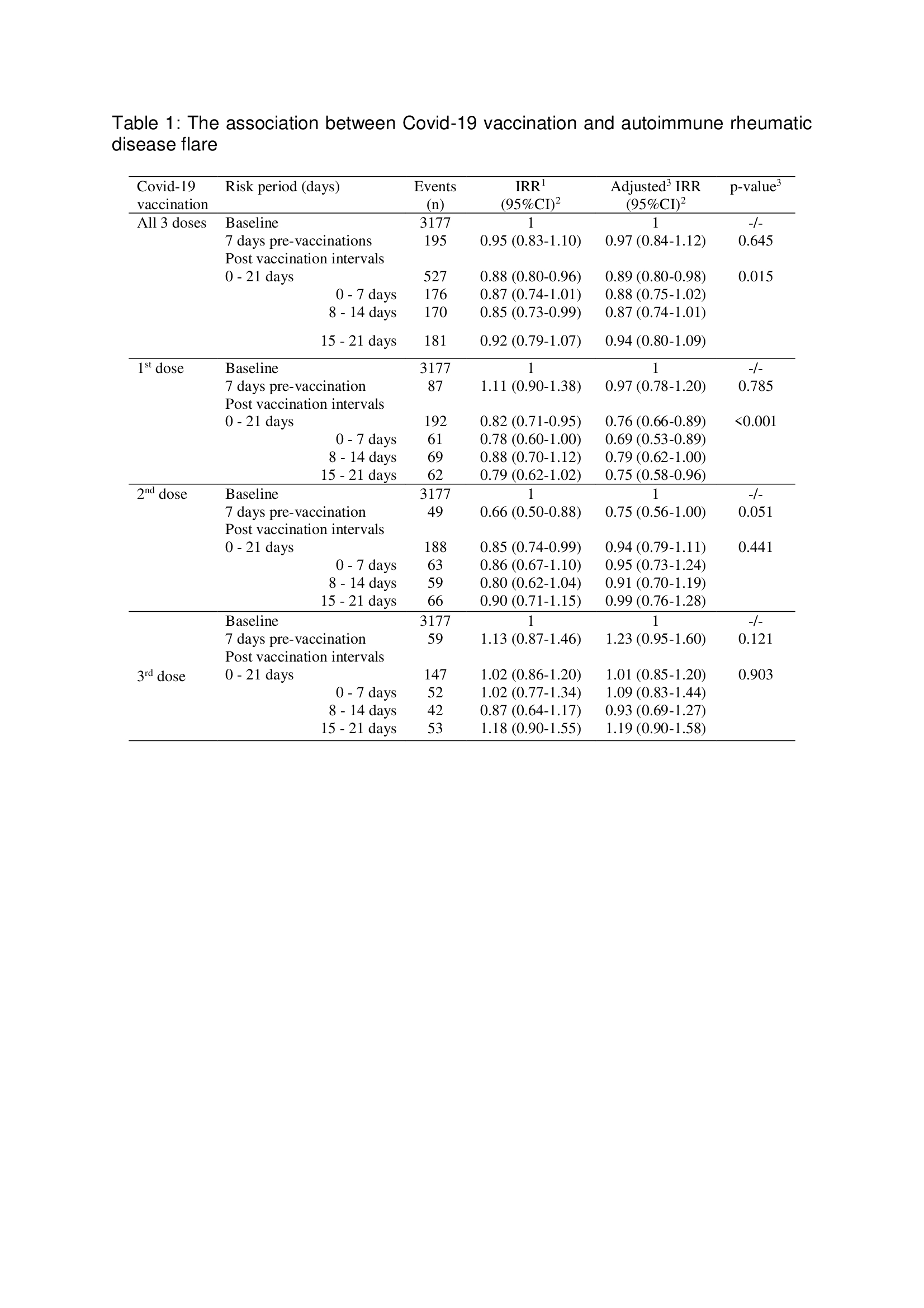 1:Incidence rate ratio, 2:95% confidence interval, 3:adjusted for four seasons.
1:Incidence rate ratio, 2:95% confidence interval, 3:adjusted for four seasons.
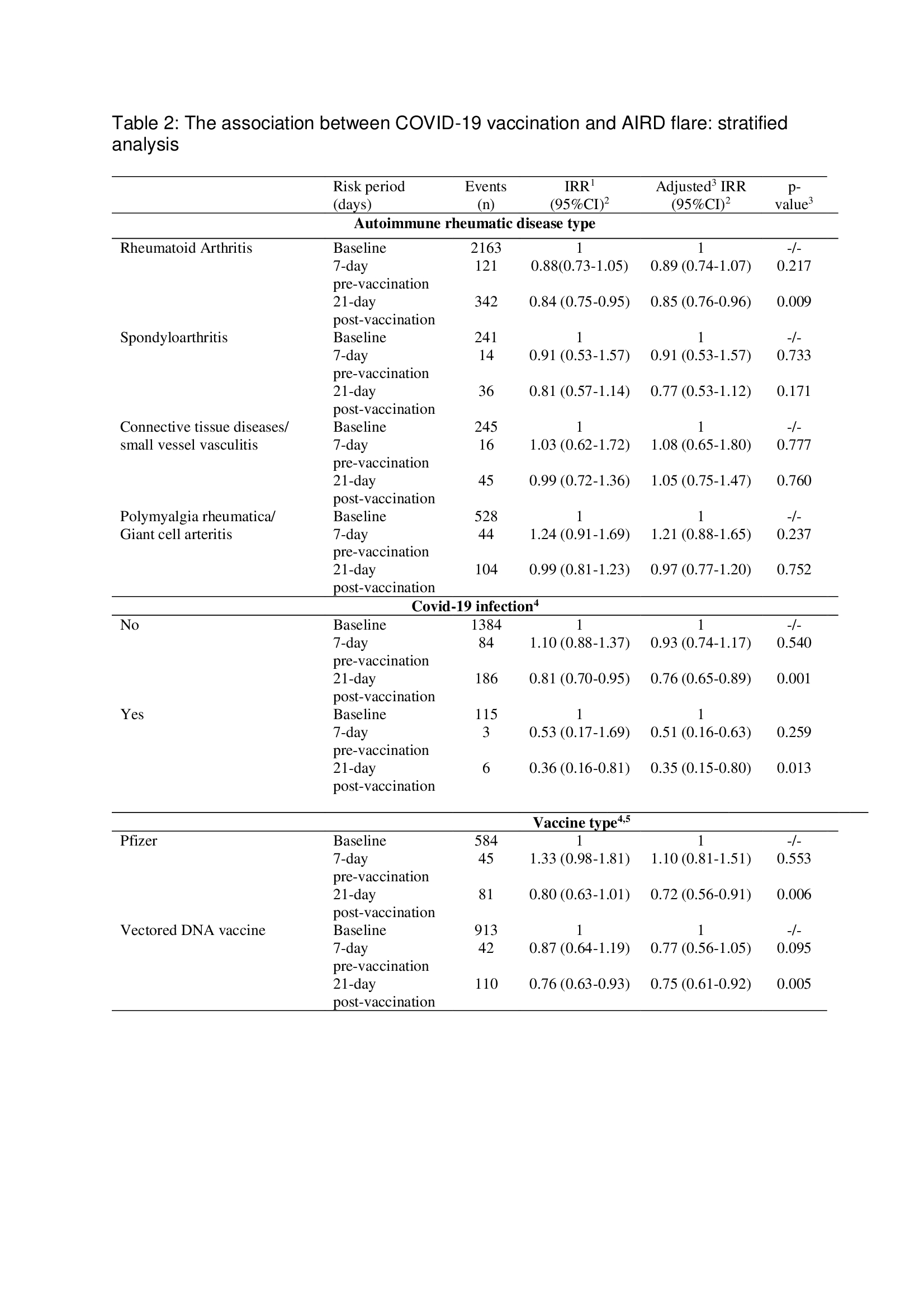 1:Incidence rate ratio, 2:95% confidence interval, 3:adjusted for four seasons, 4:First vaccine analysed. 5:People vaccinated with mRNA-1273 vaccine (n=3) were excluded from this analysis.
1:Incidence rate ratio, 2:95% confidence interval, 3:adjusted for four seasons, 4:First vaccine analysed. 5:People vaccinated with mRNA-1273 vaccine (n=3) were excluded from this analysis.
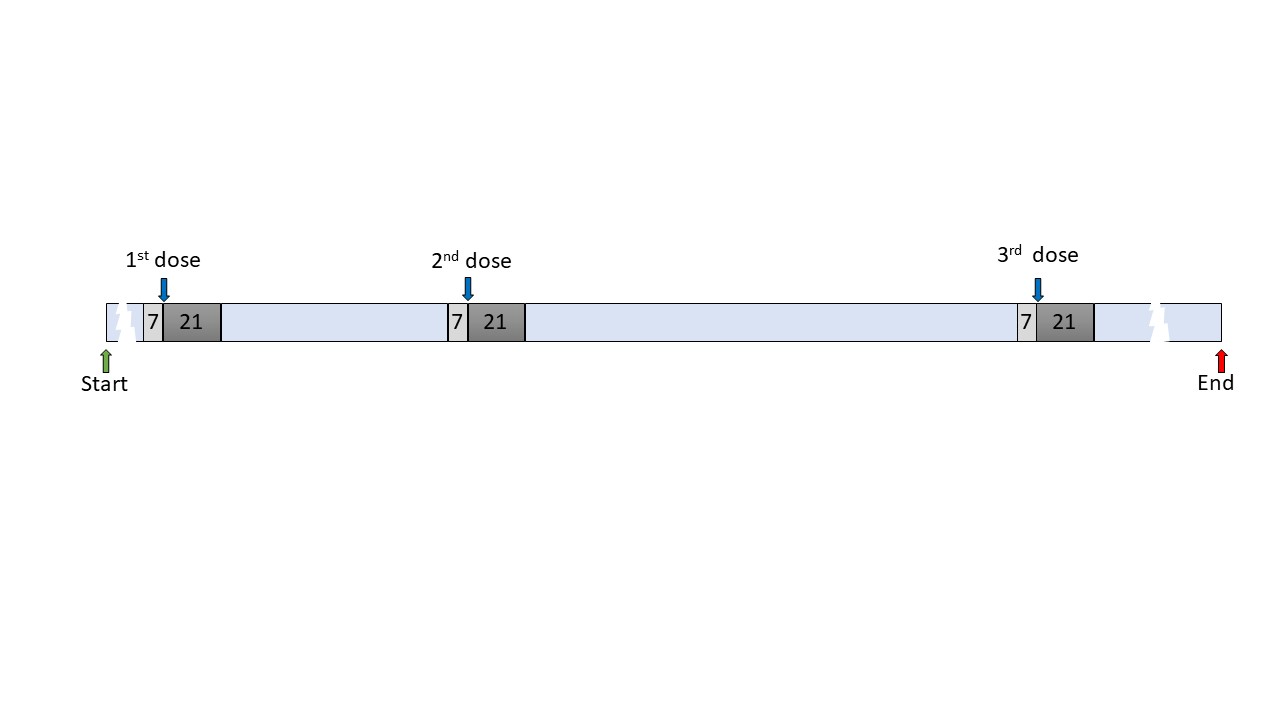 Figure 1. Schematic representation of self-controlled case series (SCCS) analysis periods. The vaccine-exposed (21-days post vaccination), pre-vaccination induction periods (7-days before vaccination) and vaccine-unexposed (the remaining vaccine-unexposed period) are shaded dark grey, light grey, light blue respectively. Vaccinations against COVID-19 are represented by dark blue arrows. Green and red arrows indicate the start and end of the study period. Not all participants received all three vaccinations. Follow up began on the latest of current registration date in GP surgery or 1st December 2020 and was censored on the earliest of 31st December 2021, death date, transfer out date, date of last data collection from the GP surgery.
Figure 1. Schematic representation of self-controlled case series (SCCS) analysis periods. The vaccine-exposed (21-days post vaccination), pre-vaccination induction periods (7-days before vaccination) and vaccine-unexposed (the remaining vaccine-unexposed period) are shaded dark grey, light grey, light blue respectively. Vaccinations against COVID-19 are represented by dark blue arrows. Green and red arrows indicate the start and end of the study period. Not all participants received all three vaccinations. Follow up began on the latest of current registration date in GP surgery or 1st December 2020 and was censored on the earliest of 31st December 2021, death date, transfer out date, date of last data collection from the GP surgery.
Disclosures: G. Nakafero, None; M. Grainge, None; T. Card, None; C. Mallen, None; J. Nguyen van Tam, None; H. Williams, None; A. Abhishek, AstraZeneca, Pfizer, Oxford immunotec, Inflazome, NGMBiopharmaceuticals, Uptodate, Cadilla pharmaceuticals.
Background/Purpose: It is unclear whether autoimmune rheumatic disease (AIRD) flares are associated with the recent prior Covid-19 vaccination, and whether the association was influenced by vaccine modality and prior Covid-19 infection. The objectives of this study were to investigate the association between vaccination against Covid-19 and AIRD flare and whether the association varied for sequential vaccinations, according to types of AIRDs, prior Covid-19, and between mRNA and vectored DNA vaccines.
Methods: We employed self-controlled case-series analysis, a method that is extensively used in vaccine safety studies because it takes between person confounding into account. Patients with AIRDs vaccinated against Covid-19 who consulted for disease flare between 01/12/2020 and 31/12/2021 were ascertained from the Clinical Practice Research Datalink (Aurum). AIRD flare was defined as consultation for AIRD with corticosteroid prescription on the same day or the next day. Vaccination was defined using date of vaccination and product code. The observation period was partitioned into vaccine-exposed (21-days after vaccination), pre-vaccination (7-days before vaccination), and remaining vaccine-unexposed periods (Figure 1). Participants contributed data with multiple vaccinations and outcomes. Season adjusted incidence rate ratios (aIRR) and 95% confidence intervals (CI) were calculated to compare the frequency of AIRD flare in different periods.
Results: Data for 3554 AIRD cases, 72% female, mean age 65 years, and 68.3% with rheumatoid arthritis were included. Covid-19 vaccination was associated with significantly fewer AIRD flares in the 21-day vaccine-exposed period when all vaccinations were considered (aIRR(95%CI) 0.89(0.80-0.98)). Using dose-stratified analyses there was a statistically significant negative association in 21-days after first Covid-19 vaccination but no association after the second or third Covid-19 vaccinations (aIRR(95%CI) 0.76(0.66-0.89), 0.94(0.79-1.11) and 1.01(0.85-1.20) respectively) (Table 1). On AIRD type stratified analyses, vaccination was not associated with disease flares. Vaccination without or after SARS-CoV-2 infection, and with vectored DNA or mRNA vaccines associated with comparable reduced risk of AIRD flares in the vaccine-exposed period after first Covid-19 vaccination (Table 2).
Conclusion: Vaccination against Covid-19 was not associated with increased AIRD flare, and vaccination with or without prior Covid-19, and with either mRNA or vectored DNA vaccines were not associated with AIRD flares. These data should address the apprehension of disease specific adverse effects from Covid-19 vaccination, an important reason for vaccine hesitancy in AIRDs, that may become even more significant as a barrier against vaccination as the perceived benefit from booster vaccination reduces.
 1:Incidence rate ratio, 2:95% confidence interval, 3:adjusted for four seasons.
1:Incidence rate ratio, 2:95% confidence interval, 3:adjusted for four seasons.  1:Incidence rate ratio, 2:95% confidence interval, 3:adjusted for four seasons, 4:First vaccine analysed. 5:People vaccinated with mRNA-1273 vaccine (n=3) were excluded from this analysis.
1:Incidence rate ratio, 2:95% confidence interval, 3:adjusted for four seasons, 4:First vaccine analysed. 5:People vaccinated with mRNA-1273 vaccine (n=3) were excluded from this analysis.  Figure 1. Schematic representation of self-controlled case series (SCCS) analysis periods. The vaccine-exposed (21-days post vaccination), pre-vaccination induction periods (7-days before vaccination) and vaccine-unexposed (the remaining vaccine-unexposed period) are shaded dark grey, light grey, light blue respectively. Vaccinations against COVID-19 are represented by dark blue arrows. Green and red arrows indicate the start and end of the study period. Not all participants received all three vaccinations. Follow up began on the latest of current registration date in GP surgery or 1st December 2020 and was censored on the earliest of 31st December 2021, death date, transfer out date, date of last data collection from the GP surgery.
Figure 1. Schematic representation of self-controlled case series (SCCS) analysis periods. The vaccine-exposed (21-days post vaccination), pre-vaccination induction periods (7-days before vaccination) and vaccine-unexposed (the remaining vaccine-unexposed period) are shaded dark grey, light grey, light blue respectively. Vaccinations against COVID-19 are represented by dark blue arrows. Green and red arrows indicate the start and end of the study period. Not all participants received all three vaccinations. Follow up began on the latest of current registration date in GP surgery or 1st December 2020 and was censored on the earliest of 31st December 2021, death date, transfer out date, date of last data collection from the GP surgery.Disclosures: G. Nakafero, None; M. Grainge, None; T. Card, None; C. Mallen, None; J. Nguyen van Tam, None; H. Williams, None; A. Abhishek, AstraZeneca, Pfizer, Oxford immunotec, Inflazome, NGMBiopharmaceuticals, Uptodate, Cadilla pharmaceuticals.

