Back
Abstract Session
Session: Abstracts: Pediatric Rheumatology – Clinical II: JIA (2209–2214)
2212: Long-term Efficacy and Safety of Subcutaneous Tocilizumab in Patients with Polyarticular or Systemic Juvenile Idiopathic Arthritis – an Extension Study of 2 Phase 1b Clinical Trials
Monday, November 14, 2022
3:45 PM – 3:55 PM Eastern Time
Location: Room 119

Hermine I. Brunner, MD, MSc, MBA
Cincinnati Children's Hospital Medical Center
Cincinnati, OH, United States
Presenting Author(s)
Hermine Brunner1, Athimalaipet Ramanan2, Gerd Horneff3, Kirsten Minden4, Inmaculada Calvo Penades5, Mauro Zucchetto6, Laura Brockwell7, Oliver Gordon7 and Fabrizio De Benedetti8, 1Division of Rheumatology, Cincinnati Children's Hospital Medical Center, University of Cincinnati, Department of Pediatrics, Cincinnati, OH, 2Bristol Royal Hospital for Children, Bristol, United Kingdom, 3Pediatrics, Asklepios Klinik Sankt Augustin GmbH, Sankt Augustin, Germany, 4Charité Universitätsmedizin Berlin, Berlin, Germany, 5Pediatric Rheumatology Unit, Hospital Universitario y Politécnico La Fe, València, Spain, 6Parexel International, Milano, Italy, 7Roche Products Ltd, Welwyn Garden City, United Kingdom, 8Division of Rheumatology, IRCCS Ospedale Pediatrico Bambino Gesu', Rome, Italy
Background/Purpose: Dosing regimens for subcutaneous tocilizumab (SC-TCZ) in patients with polyarticular-course or systemic juvenile idiopathic arthritis (pJIA or sJIA) were determined for SC-TCZ in 2 phase 1b trials by bridging to data from phase 3 trials of intravenous tocilizumab (IV-TCZ). Patients who completed these SC-TCZ trials could enter a long-term safety and efficacy extension (LTE) study. Results from this LTE are reported.
Methods: Patients with pJIA or sJIA who completed 52 weeks of open-label (OL) SC-TCZ treatment (pJIA: 162 mg every 3 weeks [Q3W] if < 30 kg or Q2W if ≥30 kg; sJIA: 162 mg Q2W or every 10 days [Q10D] if < 30 kg or Q7D if ≥30 kg) in the phase 1b trials, had an adequate response to SC-TCZ, and met additional eligibility criteria could enter the LTE. Upon entering the LTE, patients continued receiving OL SC-TCZ 162 mg dosed per body weight and JIA category. Safety was assessed for up to 5 years; efficacy, pharmacokinetics and pharmacodynamics were assessed for up to 3 years. Per protocol, patient participation in the study continued until TCZ became commercially available in a country/region or for a maximum of 5 years.
Results: Overall, 44 patients with pJIA (< 30 kg, n=24; ≥30 kg, n=20) and 38 patients with sJIA (< 30 kg, n=19; ≥30 kg, n=19) entered the LTE and 19 (43%) pJIA patients and 6 (16%) sJIA patients completed the study; most patients discontinued to start commercial TCZ and 2 pJIA patients discontinued for safety reasons (adverse events). Median treatment duration was 4.6 years for pJIA patients and 3.4 years for sJIA patients. Measurement of TCZ serum levels showed that drug exposure was maintained within ranges expected to provide a clinical benefit. Two pJIA patients ≥30 kg developed anti-TCZ antibodies of neutralizing potential during the LTE and 1 of them discontinued treatment due to lack of efficacy. There were no deaths, anaphylactic reactions, serious hepatic events, serious hypersensitivity reactions, serious bleeding events, gastrointestinal perforations, opportunistic infections, malignancies, cases of macrophage activation syndrome, or drug reactions with eosinophilia or systemic symptoms (DRESS) syndrome. Rates of adverse events (AEs) per 100 patient-years were consistent across groups (Table 1). The most common type of AEs were infections; 6 patients had serious infections (5 pJIA; 1 sJIA). 17 patients had neutropenia AEs (9 pJIA [6 grade 3, 1 grade 4]; 8 sJIA [3 grade 3]); no serious infections were observed within 30 days of neutropenia. Alanine aminotransferase or aspartate aminotransferase elevations of grade ≥3 were experienced by one sJIA patient in the context of Epstein-Barr virus infection. SC-TCZ maintained long-term control of JIA activity based on JADAS-71 (Figure). Most patients entered the LTE with inactive disease and the proportions with inactive disease and in clinical remission generally remained stable over time across all groups (Table 2).
Conclusion: SC-TCZ treatment in pJIA and sJIA patients at approved doses was well tolerated for up to 5 years and no new safety signals were identified with long-term treatment. TCZ-SC treatment resulted in well-controlled pJIA and sJIA over the 3-year assessment period.
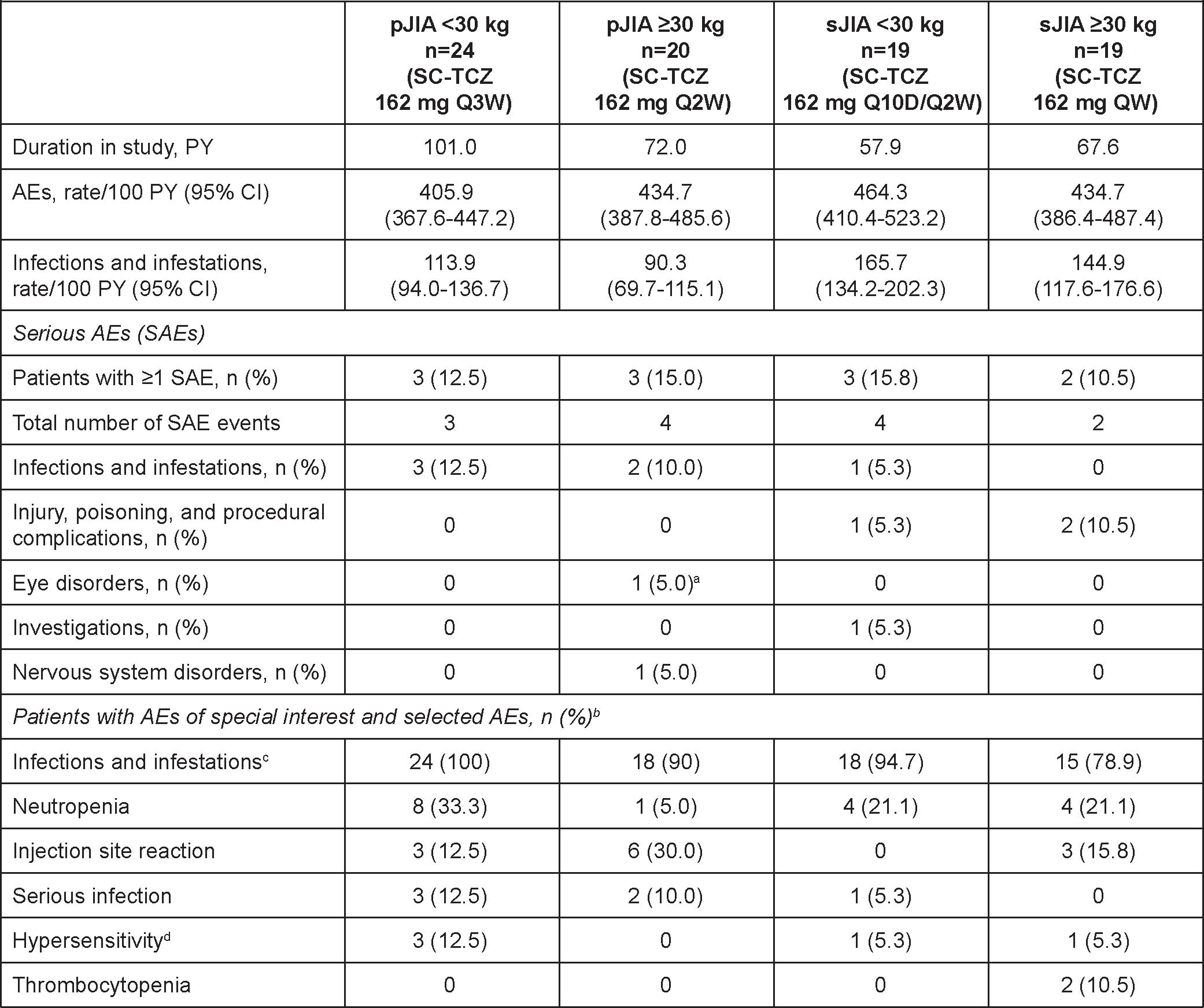 Table 1. Key Safety Outcomes.
Table 1. Key Safety Outcomes.
PY, patient-year.
aOne event of eye pain reported in conjunction with headache (definitive diagnosis could not be made). bThere were no anaphylactic reactions, serious bleeding events, demyelination events, gastrointestinal perforations, serious hepatic events, serious hypersensitivity events, malignancies, myocardial infarctions, opportunistic infections, or strokes. One patient was diagnosed with latent tuberculosis and received a full course of isoniazid. cIncludes serious and nonserious. dHypersensitivity was conservatively defined as any AEs occurring within 24 hours of TCZ treatment and not considered “unrelated to” study medication by the investigator, regardless of whether it was clinically consistent with hypersensitivity reaction.
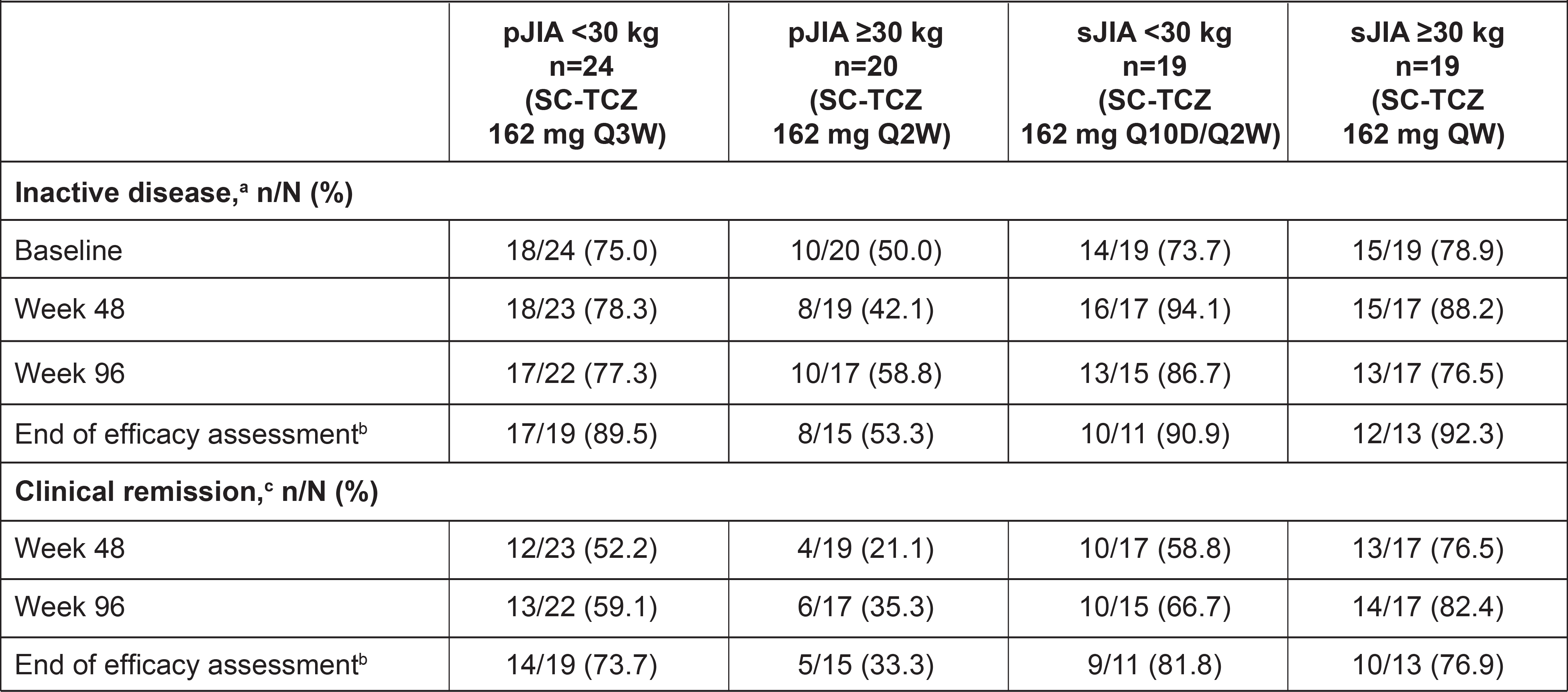 Table 2. Proportions of Patients With Inactive Disease and Clinical Remission.
Table 2. Proportions of Patients With Inactive Disease and Clinical Remission.
aInactive disease was defined according to Wallace criteria as no presence of active joints, absence of uveitis, no fever, rash, serositis, splenomegaly, hepatomegaly, or generalized lymphadenopathy attributable to pJIA or sJIA, erythrocyte sedimentation rate < 20 mm/h, physician global visual analog scale score ≤10 mm, and duration of morning stiffness ≤15 minutes.
bWeek 156 for pJIA, week 152 for sJIA.
cClinical remission was defined as inactive disease for a minimum of 6 continuous months irrespective of disease-modifying antirheumatic drug, nonsteroidal anti-inflammatory drug, or corticosteroid use.
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/17574/QHOPTGBB-1274978-3-ANY.jpg width=440 height=580.752688172043 border=0 style=border-style: none;>
Disclosures: H. Brunner, Cincinnati Children's Hospital, Pfizer, GlaxoSmithKlein(GSK), AbbVie/Abbott, AstraZeneca, Medimmune, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, EMD Serono, Idorsia, Cerecor, Janssen, Roche, Merck/MSD, Novartis, R-Harm, Sanofi; A. Ramanan, Roche, Eli Lilly, AbbVie/Abbott, Novartis, UCB, Sobi; G. Horneff, Roche, Pfizer, Novartis, Merck/MSD, Eli Lilly, AbbVie/Abbott; K. Minden, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Roche, Biogen; I. Calvo Penades, Novartis, GlaxoSmithKlein(GSK), Ipsen, AbbVie/Abbott, Sobi; M. Zucchetto, Parexel International; L. Brockwell, Roche; O. Gordon, Roche, Novartis; F. De Benedetti, Sobi, Novimmune, Pfizer, Novartis, Roche, Abbvie.
Background/Purpose: Dosing regimens for subcutaneous tocilizumab (SC-TCZ) in patients with polyarticular-course or systemic juvenile idiopathic arthritis (pJIA or sJIA) were determined for SC-TCZ in 2 phase 1b trials by bridging to data from phase 3 trials of intravenous tocilizumab (IV-TCZ). Patients who completed these SC-TCZ trials could enter a long-term safety and efficacy extension (LTE) study. Results from this LTE are reported.
Methods: Patients with pJIA or sJIA who completed 52 weeks of open-label (OL) SC-TCZ treatment (pJIA: 162 mg every 3 weeks [Q3W] if < 30 kg or Q2W if ≥30 kg; sJIA: 162 mg Q2W or every 10 days [Q10D] if < 30 kg or Q7D if ≥30 kg) in the phase 1b trials, had an adequate response to SC-TCZ, and met additional eligibility criteria could enter the LTE. Upon entering the LTE, patients continued receiving OL SC-TCZ 162 mg dosed per body weight and JIA category. Safety was assessed for up to 5 years; efficacy, pharmacokinetics and pharmacodynamics were assessed for up to 3 years. Per protocol, patient participation in the study continued until TCZ became commercially available in a country/region or for a maximum of 5 years.
Results: Overall, 44 patients with pJIA (< 30 kg, n=24; ≥30 kg, n=20) and 38 patients with sJIA (< 30 kg, n=19; ≥30 kg, n=19) entered the LTE and 19 (43%) pJIA patients and 6 (16%) sJIA patients completed the study; most patients discontinued to start commercial TCZ and 2 pJIA patients discontinued for safety reasons (adverse events). Median treatment duration was 4.6 years for pJIA patients and 3.4 years for sJIA patients. Measurement of TCZ serum levels showed that drug exposure was maintained within ranges expected to provide a clinical benefit. Two pJIA patients ≥30 kg developed anti-TCZ antibodies of neutralizing potential during the LTE and 1 of them discontinued treatment due to lack of efficacy. There were no deaths, anaphylactic reactions, serious hepatic events, serious hypersensitivity reactions, serious bleeding events, gastrointestinal perforations, opportunistic infections, malignancies, cases of macrophage activation syndrome, or drug reactions with eosinophilia or systemic symptoms (DRESS) syndrome. Rates of adverse events (AEs) per 100 patient-years were consistent across groups (Table 1). The most common type of AEs were infections; 6 patients had serious infections (5 pJIA; 1 sJIA). 17 patients had neutropenia AEs (9 pJIA [6 grade 3, 1 grade 4]; 8 sJIA [3 grade 3]); no serious infections were observed within 30 days of neutropenia. Alanine aminotransferase or aspartate aminotransferase elevations of grade ≥3 were experienced by one sJIA patient in the context of Epstein-Barr virus infection. SC-TCZ maintained long-term control of JIA activity based on JADAS-71 (Figure). Most patients entered the LTE with inactive disease and the proportions with inactive disease and in clinical remission generally remained stable over time across all groups (Table 2).
Conclusion: SC-TCZ treatment in pJIA and sJIA patients at approved doses was well tolerated for up to 5 years and no new safety signals were identified with long-term treatment. TCZ-SC treatment resulted in well-controlled pJIA and sJIA over the 3-year assessment period.
 Table 1. Key Safety Outcomes.
Table 1. Key Safety Outcomes.PY, patient-year.
aOne event of eye pain reported in conjunction with headache (definitive diagnosis could not be made). bThere were no anaphylactic reactions, serious bleeding events, demyelination events, gastrointestinal perforations, serious hepatic events, serious hypersensitivity events, malignancies, myocardial infarctions, opportunistic infections, or strokes. One patient was diagnosed with latent tuberculosis and received a full course of isoniazid. cIncludes serious and nonserious. dHypersensitivity was conservatively defined as any AEs occurring within 24 hours of TCZ treatment and not considered “unrelated to” study medication by the investigator, regardless of whether it was clinically consistent with hypersensitivity reaction.
 Table 2. Proportions of Patients With Inactive Disease and Clinical Remission.
Table 2. Proportions of Patients With Inactive Disease and Clinical Remission.aInactive disease was defined according to Wallace criteria as no presence of active joints, absence of uveitis, no fever, rash, serositis, splenomegaly, hepatomegaly, or generalized lymphadenopathy attributable to pJIA or sJIA, erythrocyte sedimentation rate < 20 mm/h, physician global visual analog scale score ≤10 mm, and duration of morning stiffness ≤15 minutes.
bWeek 156 for pJIA, week 152 for sJIA.
cClinical remission was defined as inactive disease for a minimum of 6 continuous months irrespective of disease-modifying antirheumatic drug, nonsteroidal anti-inflammatory drug, or corticosteroid use.
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/17574/QHOPTGBB-1274978-3-ANY.jpg width=440 height=580.752688172043 border=0 style=border-style: none;>
Figure. JADAS-71 over time in patients with pJIA (A) and sJIA (B).
IQR, interquartile range; JADAS-71, Juvenile Arthritis Disease Activity Score including 71 joints.
Dashed horizontal lines represent thresholds for high disease activity (>10.5), moderate disease activity (3.9-10.5), low disease activity (≤3.8), and inactive disease (≤1).
IQR, interquartile range; JADAS-71, Juvenile Arthritis Disease Activity Score including 71 joints.
Dashed horizontal lines represent thresholds for high disease activity (>10.5), moderate disease activity (3.9-10.5), low disease activity (≤3.8), and inactive disease (≤1).
Disclosures: H. Brunner, Cincinnati Children's Hospital, Pfizer, GlaxoSmithKlein(GSK), AbbVie/Abbott, AstraZeneca, Medimmune, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, EMD Serono, Idorsia, Cerecor, Janssen, Roche, Merck/MSD, Novartis, R-Harm, Sanofi; A. Ramanan, Roche, Eli Lilly, AbbVie/Abbott, Novartis, UCB, Sobi; G. Horneff, Roche, Pfizer, Novartis, Merck/MSD, Eli Lilly, AbbVie/Abbott; K. Minden, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Roche, Biogen; I. Calvo Penades, Novartis, GlaxoSmithKlein(GSK), Ipsen, AbbVie/Abbott, Sobi; M. Zucchetto, Parexel International; L. Brockwell, Roche; O. Gordon, Roche, Novartis; F. De Benedetti, Sobi, Novimmune, Pfizer, Novartis, Roche, Abbvie.

