Back
Poster Session B
Session: (0752–0778) Immunological Complications of Medical Therapy Poster
0764: No Impact of Prior DMARD Exposures on Mortality in US Veterans with Cancer Treated with Immune Checkpoint Inhibitors
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- TB
Tawnie Braaten, MD
UNIVERSITY OF UTAH
SALT LAKE CITY, UT, United States
Abstract Poster Presenter(s)
Tawnie Braaten1, Brian Sauer2, Gary Kunkel3, Jessica Walsh3, Jorge Rojas4, Bryant England5, Joshua Baker6 and Grant Cannon7, 1University of Utah Hospitals and Clinics and VA Salt Lake City Health Care System, Salt Lake City, UT, 2Salt Lake City VA/University of Utah, Salt Lake City, UT, 3University of Utah, Salt Lake City, UT, 4George E. Wahlen Department of Veterans Affairs Salt Lake City Health Care System, Salt Lake City, UT, 5University of Nebraska Medical Center, Omaha, NE, 6University of Pennsylvania and Corporal Michael J. Crescenz VA Medical Center, Philadelphia, 7Salt Lake City VA, Salt Lake city
Background/Purpose: Immune checkpoint inhibitor (ICI) use for the treatment of multiple cancers continues to expand. Data on ICI treatment in autoimmune disease is limited as these patients were excluded from most clinical trials. Concern has been raised that the use of DMARDs prior to receiving ICI may complicate the immunomodulatory dynamic having a negative impact on the effectiveness of ICIs. We compared 2-year all-cause mortality between patients exposed or unexposed to DMARD therapy prior to ICI treatment in the VA national healthcare system.
Methods: We performed a retrospective cohort study of patients receiving ICIs within the Veterans Health Administration (VHA) between 2011-2022. Patients were included if they received ICI therapy between 2011-2020, allowing for 2 years of follow up. Data were obtained from the Corporate Data Warehouse (CDW) for pharmacy and demographic information, VA cancer registry for cancer diagnosis, and vital status for all-cause mortality. For each DMARD, a continuous course was defined as multiple sequential dispenses without a 90-day gap in therapy. The most proximate DMARD course prior to the beginning of ICI treatment was identified for each DMARD. Status for each proximate DMARD prior to ICI treatment was classified into one of 3 groups based on the end date for the course: 1) continued up to or within 90 days prior to ICI start, 2) terminated within 91-365 days, or 3) terminated >365 days prior to ICI treatment. DMARDs were categorized as csDMARD or biologic/targeted synthetic DMARD (b/tsDMARD), defined in Table 2. All-cause mortality for all cancers and lung cancer (the most common malignancy) subset was compared based on the relationship of DMARD continuation/termination prior to initiating ICIs.
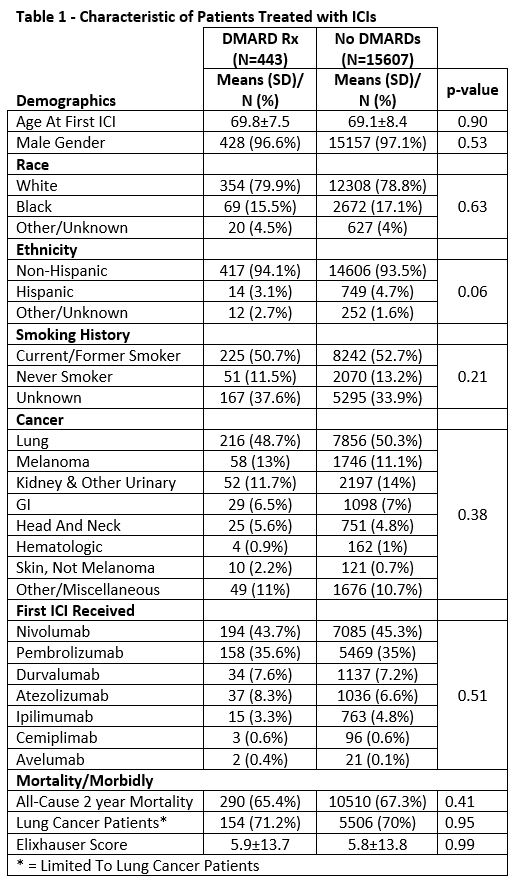
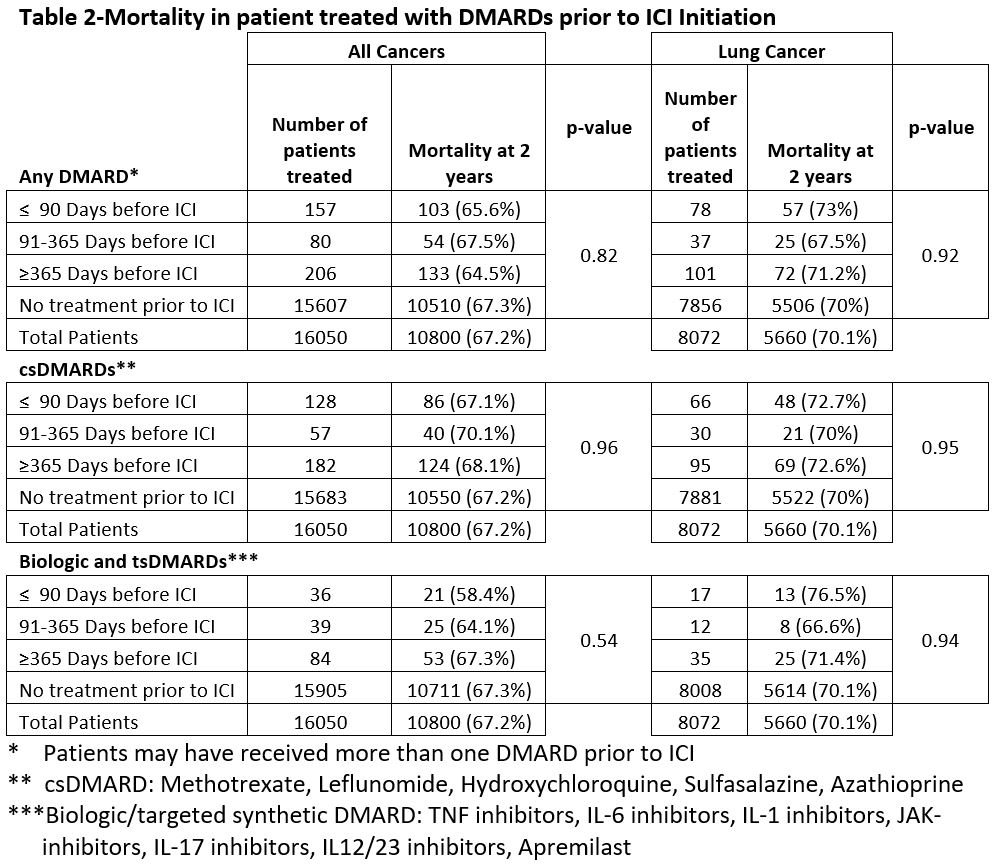
Results: There were 16,050 veterans who received ICI treatment during the study period with 443 (2.7%) patients previously receiving a DMARD. There were no significant differences in age, gender, race, ethnicity, smoking history, cancer type or first ICI treatment type between those previously exposed and those never exposed to a DMARD (Table 1). Lung cancer was the most common malignancy in both groups at 48.7% and 50.3% (Table 1). In the 443 patients exposed to DMARDs, 157 (35.4%) patients had received a DMARD within 90 days of ICI start, 80 (18.4%) had discontinued DMARD between 91-365 days prior to ICI, and 206 (47.5%) discontinued DMARDs >1 year. The 2-year mortality in these groups was 65.6%, 67.5%, and 64.5% respectively compared to 67.3% in those not exposed to DMARDs (p= 0.82). In the csDMARD groups 2-year mortality was 67.1%, 70.1%, and 68.1% vs 67.2% in those not exposed to csDMARDs (Table 2). In the biologic/ts DMARD groups mortality was 58.5%, 64.1%, 67.3%. vs 67.3% in those not exposed to b/ts DMARDs (Table 2). Sub analyses limited to lung cancer patients produced similar findings.
Conclusion: Exposure to DMARDs prior to ICI treatment was not associated with 2-year crude mortality rates compared to patients receiving ICI treatment not previously exposed to DMARDs. These findings suggest that patients treated with DMARDs commonly used in autoimmune diseases do not have worse survival outcomes during subsequent ICI therapy.
Disclosures: T. Braaten, Gilead; B. Sauer, None; G. Kunkel, None; J. Walsh, AbbVie, Amgen, Lilly, Novartis, Pfizer, UCB, Celgene, Janssen, Merck; J. Rojas, None; B. England, Boehringer-Ingelheim; J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; G. Cannon, None.
Background/Purpose: Immune checkpoint inhibitor (ICI) use for the treatment of multiple cancers continues to expand. Data on ICI treatment in autoimmune disease is limited as these patients were excluded from most clinical trials. Concern has been raised that the use of DMARDs prior to receiving ICI may complicate the immunomodulatory dynamic having a negative impact on the effectiveness of ICIs. We compared 2-year all-cause mortality between patients exposed or unexposed to DMARD therapy prior to ICI treatment in the VA national healthcare system.
Methods: We performed a retrospective cohort study of patients receiving ICIs within the Veterans Health Administration (VHA) between 2011-2022. Patients were included if they received ICI therapy between 2011-2020, allowing for 2 years of follow up. Data were obtained from the Corporate Data Warehouse (CDW) for pharmacy and demographic information, VA cancer registry for cancer diagnosis, and vital status for all-cause mortality. For each DMARD, a continuous course was defined as multiple sequential dispenses without a 90-day gap in therapy. The most proximate DMARD course prior to the beginning of ICI treatment was identified for each DMARD. Status for each proximate DMARD prior to ICI treatment was classified into one of 3 groups based on the end date for the course: 1) continued up to or within 90 days prior to ICI start, 2) terminated within 91-365 days, or 3) terminated >365 days prior to ICI treatment. DMARDs were categorized as csDMARD or biologic/targeted synthetic DMARD (b/tsDMARD), defined in Table 2. All-cause mortality for all cancers and lung cancer (the most common malignancy) subset was compared based on the relationship of DMARD continuation/termination prior to initiating ICIs.
Results: There were 16,050 veterans who received ICI treatment during the study period with 443 (2.7%) patients previously receiving a DMARD. There were no significant differences in age, gender, race, ethnicity, smoking history, cancer type or first ICI treatment type between those previously exposed and those never exposed to a DMARD (Table 1). Lung cancer was the most common malignancy in both groups at 48.7% and 50.3% (Table 1). In the 443 patients exposed to DMARDs, 157 (35.4%) patients had received a DMARD within 90 days of ICI start, 80 (18.4%) had discontinued DMARD between 91-365 days prior to ICI, and 206 (47.5%) discontinued DMARDs >1 year. The 2-year mortality in these groups was 65.6%, 67.5%, and 64.5% respectively compared to 67.3% in those not exposed to DMARDs (p= 0.82). In the csDMARD groups 2-year mortality was 67.1%, 70.1%, and 68.1% vs 67.2% in those not exposed to csDMARDs (Table 2). In the biologic/ts DMARD groups mortality was 58.5%, 64.1%, 67.3%. vs 67.3% in those not exposed to b/ts DMARDs (Table 2). Sub analyses limited to lung cancer patients produced similar findings.
Conclusion: Exposure to DMARDs prior to ICI treatment was not associated with 2-year crude mortality rates compared to patients receiving ICI treatment not previously exposed to DMARDs. These findings suggest that patients treated with DMARDs commonly used in autoimmune diseases do not have worse survival outcomes during subsequent ICI therapy.


Disclosures: T. Braaten, Gilead; B. Sauer, None; G. Kunkel, None; J. Walsh, AbbVie, Amgen, Lilly, Novartis, Pfizer, UCB, Celgene, Janssen, Merck; J. Rojas, None; B. England, Boehringer-Ingelheim; J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; G. Cannon, None.

