Back
Poster Session B
Antiphospholipid Syndrome
Session: (0671–0694) Antiphospholipid Syndrome Poster
0677: Obstructive Sleep Apnea in Primary Antiphospholipid Syndrome Impacts Damage Accrual
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

Gustavo Balbi, MD
Universidade de Sao Paulo
Sao Paulo, S�o Paulo, Brazil
Abstract Poster Presenter(s)
Gustavo Balbi1, Flavio Victor Signorelli2, Ana Paula Gandara3, Indira Azam3, Dilson Marreiros3, Luciano Drager3 and Danieli Castro Oliveira de Andrade4, 1Universidade de São Paulo, São Paulo, Brazil, 2Universidade do Estado do Rio de Janeiro, Rio De Janeiro, Brazil, 3University of São Paulo, São Paulo, Brazil, 4Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil
Background/Purpose: Obstructive sleep apnea (OSA) has been associated with an increased risk of thrombotic-related events, such as myocardial infarction and cerebrovascular diseases in the general population. Thus, understanding the impact of OSA in patients with thrombotic primary antiphospholipid syndrome (tPAPS) is important to determine potential non-traditional factors that may contribute to thrombotic recurrence in those patients. We aimed to evaluate: 1) the frequency of OSA in tPAPS; 2) the differences in APS characteristics, damage accrual measured by Damage Index for Antiphospholipid Syndrome (DIAPS) and levels of biomarkers associated with thrombosis (von Willebrand factor - vWF) between tPAPS patients with and without OSA; 3) the performance of screening tools for OSA in this scenario.
Methods: We consecutively enrolled 60 patients with tPAPS (Sydney criteria). Damage accrual was measured by DIAPS. vWF antigen levels was determined using ELISA (AbcamTM). OSA was evaluated using a portable sleep monitor (Embletta Gold, Natus Medical Inc., Ontario, Canada). OSA was defined by an apnea-hypopnea index (AHI) ≥15. Patients with and without OSA were compared regarding APS clinical and laboratory characteristics, damage accrual and vWF levels, using standard statistical procedures. For the diagnostic yield analysis, we applied four different screening tools for assessing OSA (Epworth Sleepiness Scale [ESS], Berlin questionnaire, NoSAS score [Neck circumference, Obesity, Snoring, Age, Sex], and STOP-Bang [Snoring, Tiredness, Observed apnea, Pressure (high blood), BMI, Age, Neck circumference, Gender]) and compared their performance using ROC curves.
Results: Out of 60 patients who underwent sleep monitoring, 52 were included for analysis (8 patients were excluded due to low quality of sleep data). The majority of patients were females (82.7%) and whites (76.9%). The mean age and body-mass index were 48±14 years and 31.1±6.5 Kg/m2, respectively. OSA was diagnosed in 13 patients (25.0%). Patients with OSA were older (56.9±10.7 vs. 44.6±13.8, p=0.006) and had higher neck and waist circumferences, waist-to-hip ratio and systolic blood pressure levels. Patients with OSA had a trend for previous arterial events (61.5% vs. 33.3%, p=0.073). We also found higher levels of vWF (38.9 [26.45-56.3] vs. 32.6 [20.4-38.3] mUI/mL, p=0.038) and higher DIAPS (5 [2.5-9.5] vs. 2 [1-5], p=0.020) in patients with OSA (vs. without OSA). Among screening tools, NoSAS had the highest area under ROC curve (0.806, 95% CI: 0.672-0.939, p=0.001), followed by STOP-Bang (0.772, 95% CI: 0.607-0.938, p=0.004).
Conclusion: The frequency of OSA was strikingly high in our cohort. tPAPS patients with OSA had numerically higher rates of previous arterial events and also significantly higher levels of vWF and higher damage accrual than those without OSA. NoSAS appears to be the best screening tool for OSA in patients with PAPS.
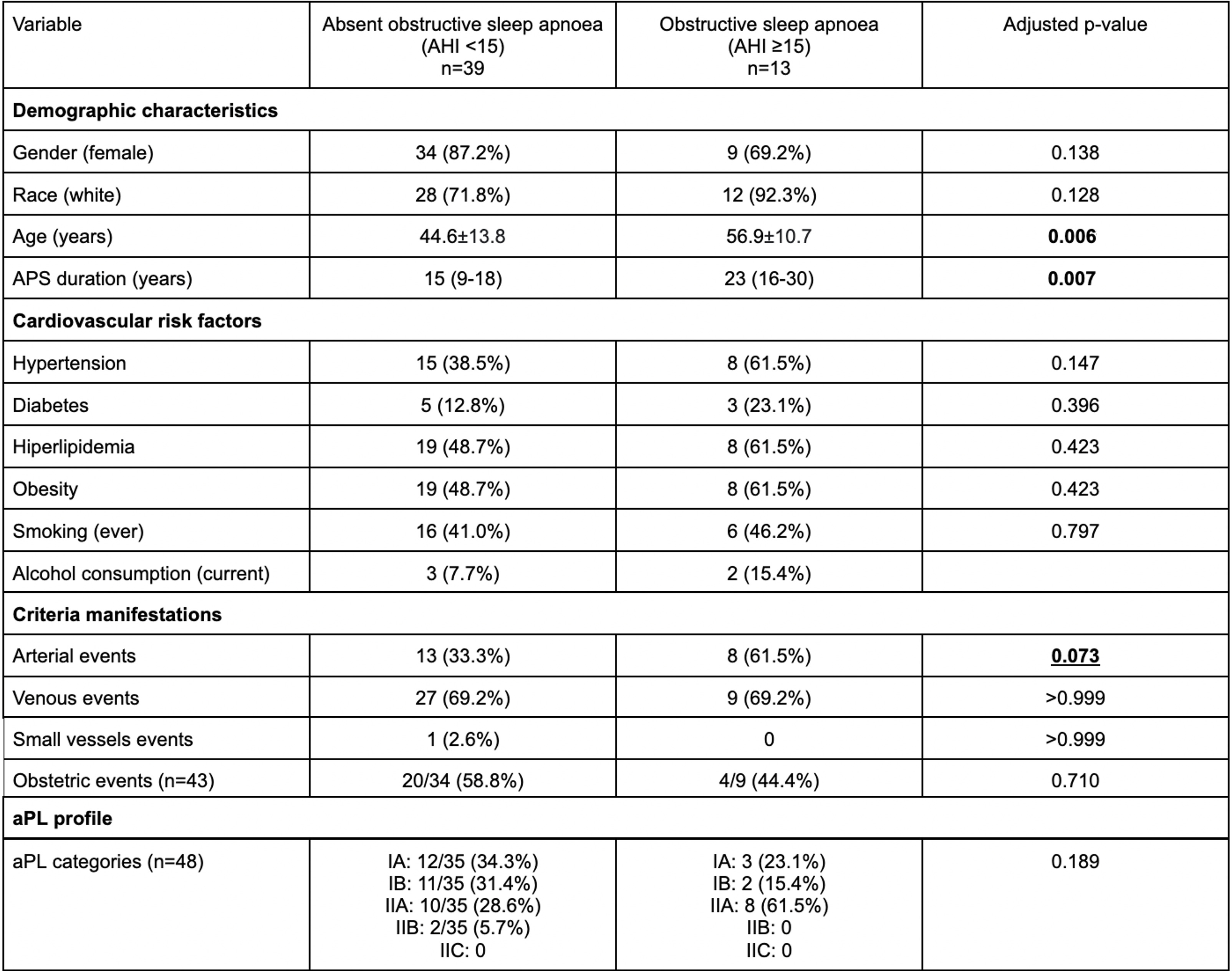 Table 1. Clinical and laboratory characteristics of PAPS patients with and without OSA.
Table 1. Clinical and laboratory characteristics of PAPS patients with and without OSA.
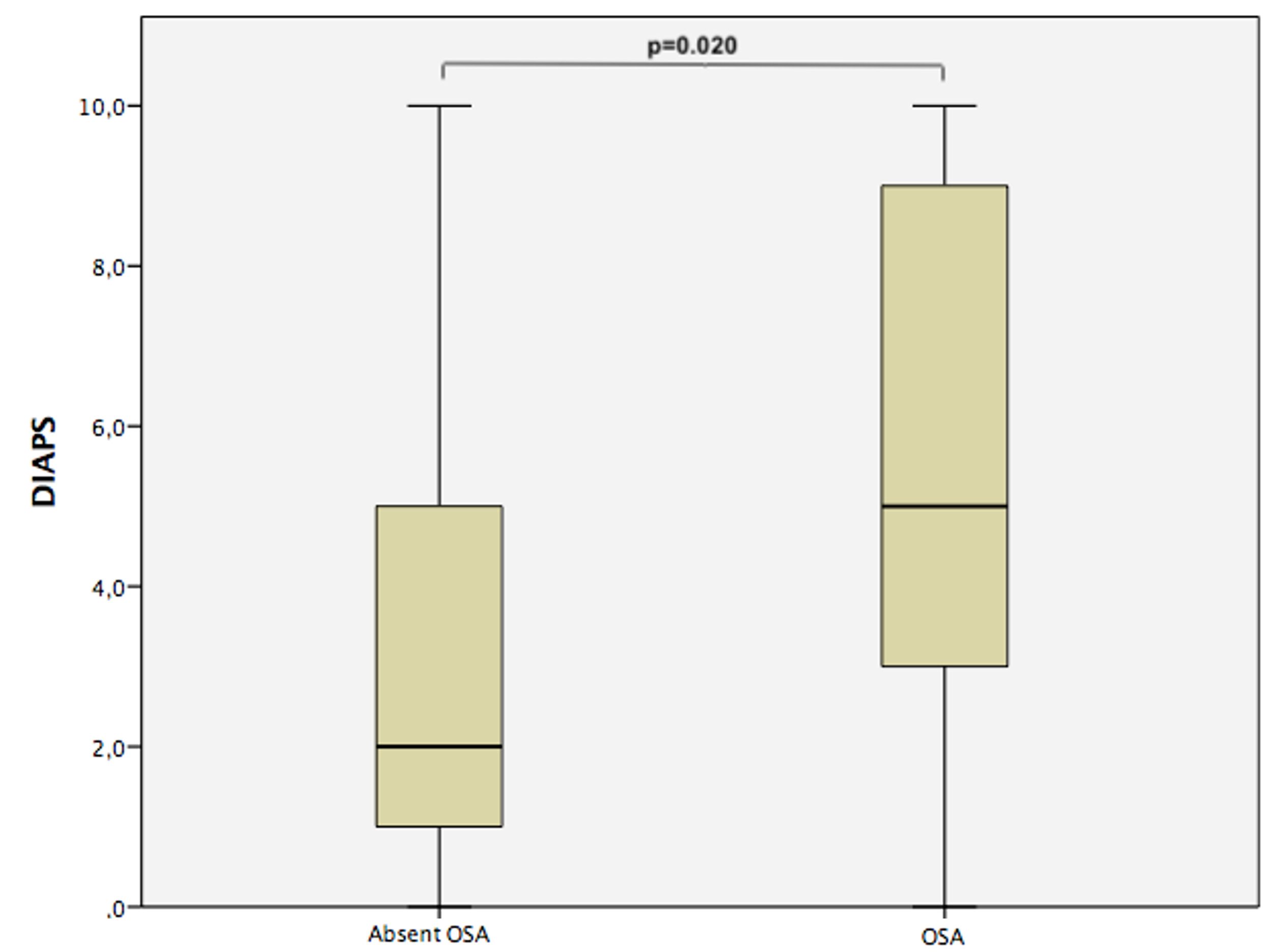 Figure 1. Damage Index for AntiPhospholipid Syndrome (DIAPS) values of PAPS patients with and without OSA.
Figure 1. Damage Index for AntiPhospholipid Syndrome (DIAPS) values of PAPS patients with and without OSA.
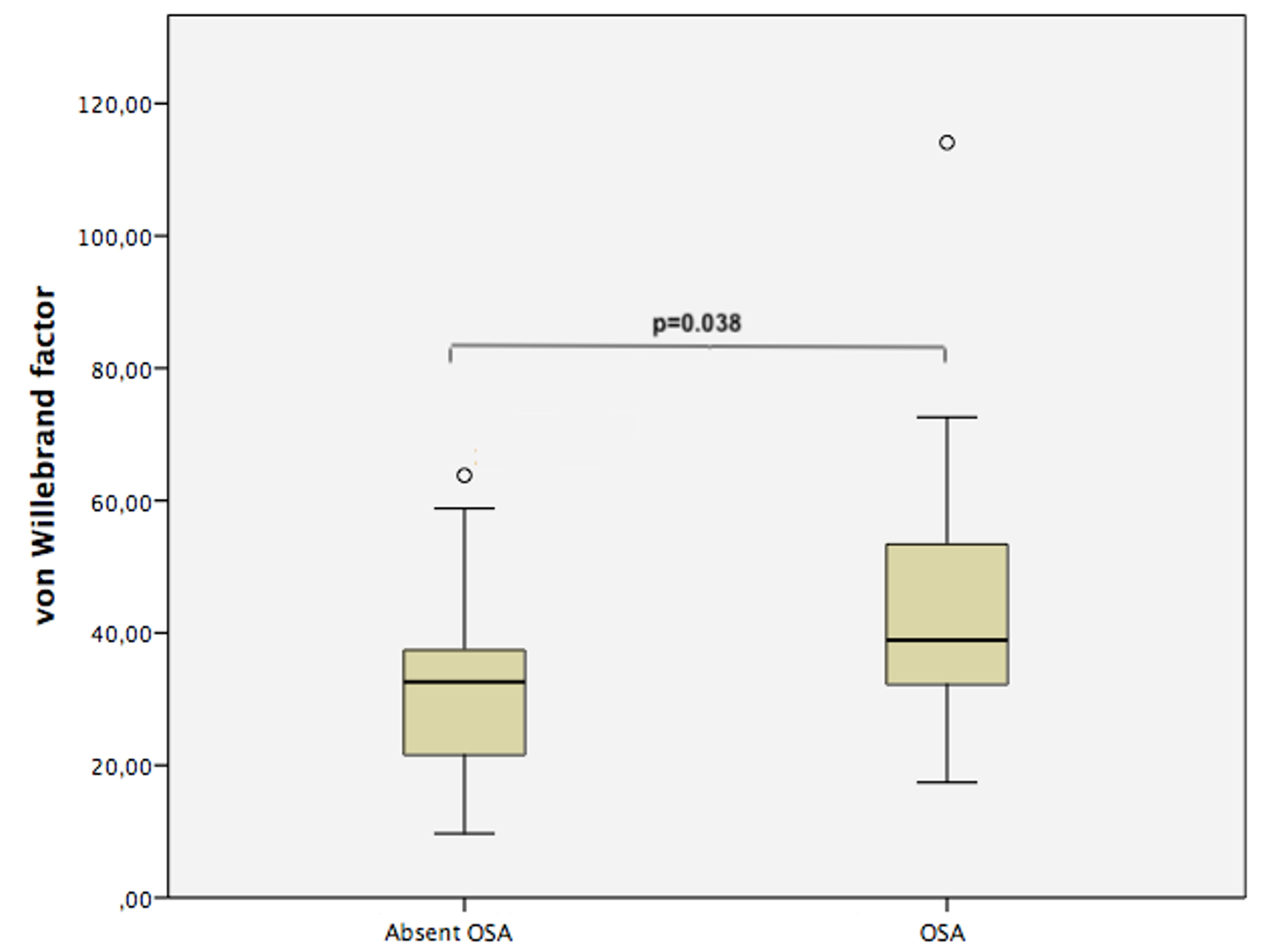 Figure 2. von Willebrand factor levels of PAPS patients with and without OSA.
Figure 2. von Willebrand factor levels of PAPS patients with and without OSA.
Disclosures: G. Balbi, None; F. Signorelli, None; A. Gandara, None; I. Azam, None; D. Marreiros, None; L. Drager, None; D. Castro Oliveira de Andrade, None.
Background/Purpose: Obstructive sleep apnea (OSA) has been associated with an increased risk of thrombotic-related events, such as myocardial infarction and cerebrovascular diseases in the general population. Thus, understanding the impact of OSA in patients with thrombotic primary antiphospholipid syndrome (tPAPS) is important to determine potential non-traditional factors that may contribute to thrombotic recurrence in those patients. We aimed to evaluate: 1) the frequency of OSA in tPAPS; 2) the differences in APS characteristics, damage accrual measured by Damage Index for Antiphospholipid Syndrome (DIAPS) and levels of biomarkers associated with thrombosis (von Willebrand factor - vWF) between tPAPS patients with and without OSA; 3) the performance of screening tools for OSA in this scenario.
Methods: We consecutively enrolled 60 patients with tPAPS (Sydney criteria). Damage accrual was measured by DIAPS. vWF antigen levels was determined using ELISA (AbcamTM). OSA was evaluated using a portable sleep monitor (Embletta Gold, Natus Medical Inc., Ontario, Canada). OSA was defined by an apnea-hypopnea index (AHI) ≥15. Patients with and without OSA were compared regarding APS clinical and laboratory characteristics, damage accrual and vWF levels, using standard statistical procedures. For the diagnostic yield analysis, we applied four different screening tools for assessing OSA (Epworth Sleepiness Scale [ESS], Berlin questionnaire, NoSAS score [Neck circumference, Obesity, Snoring, Age, Sex], and STOP-Bang [Snoring, Tiredness, Observed apnea, Pressure (high blood), BMI, Age, Neck circumference, Gender]) and compared their performance using ROC curves.
Results: Out of 60 patients who underwent sleep monitoring, 52 were included for analysis (8 patients were excluded due to low quality of sleep data). The majority of patients were females (82.7%) and whites (76.9%). The mean age and body-mass index were 48±14 years and 31.1±6.5 Kg/m2, respectively. OSA was diagnosed in 13 patients (25.0%). Patients with OSA were older (56.9±10.7 vs. 44.6±13.8, p=0.006) and had higher neck and waist circumferences, waist-to-hip ratio and systolic blood pressure levels. Patients with OSA had a trend for previous arterial events (61.5% vs. 33.3%, p=0.073). We also found higher levels of vWF (38.9 [26.45-56.3] vs. 32.6 [20.4-38.3] mUI/mL, p=0.038) and higher DIAPS (5 [2.5-9.5] vs. 2 [1-5], p=0.020) in patients with OSA (vs. without OSA). Among screening tools, NoSAS had the highest area under ROC curve (0.806, 95% CI: 0.672-0.939, p=0.001), followed by STOP-Bang (0.772, 95% CI: 0.607-0.938, p=0.004).
Conclusion: The frequency of OSA was strikingly high in our cohort. tPAPS patients with OSA had numerically higher rates of previous arterial events and also significantly higher levels of vWF and higher damage accrual than those without OSA. NoSAS appears to be the best screening tool for OSA in patients with PAPS.
 Table 1. Clinical and laboratory characteristics of PAPS patients with and without OSA.
Table 1. Clinical and laboratory characteristics of PAPS patients with and without OSA. Figure 1. Damage Index for AntiPhospholipid Syndrome (DIAPS) values of PAPS patients with and without OSA.
Figure 1. Damage Index for AntiPhospholipid Syndrome (DIAPS) values of PAPS patients with and without OSA. Figure 2. von Willebrand factor levels of PAPS patients with and without OSA.
Figure 2. von Willebrand factor levels of PAPS patients with and without OSA.Disclosures: G. Balbi, None; F. Signorelli, None; A. Gandara, None; I. Azam, None; D. Marreiros, None; L. Drager, None; D. Castro Oliveira de Andrade, None.

