Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0883–0912) RA – Diagnosis, Manifestations, and Outcomes Poster II
0911: Peer Coach Intervention to Improve Primary Cardiovascular Disease Screening for Patients with Rheumatoid Arthritis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- IN
Iris Navarro-Millán, MD
Weill Cornell Medicine, Hospital for Special Surgery
New York, NY, United States
Abstract Poster Presenter(s)
Iris Navarro-Millan1, Mackenzie Brown2, Yuliana Domínguez Páez3, Geyanne Lui4, Joan Weiner1, Shelley fritz1, Tien Sydnor-Campbell5, Aberdeen Allen6, Assem Jabri1, Shilpa Venkatachalam7, Kelly Gavigan8, William Benjamin Nowell9, Jeffrey Curtis10, Liana Fraenkel11 and Monika Safford1, 1Weill Cornell Medicine, New York, NY, 2Weill Cornell Medicine, Brooklyn, NY, 3Weill Cornell Medicine, Bronx, NY, 4Albert Einstein College of Medicine, New York, NY, 5Weill Cornell Medicine, Philedelphia, 6Weill Cornell Medicine, Parlin, 7Global Healthy Living Foundation, New York, NY, 8Global Healthy Living Foundation, Upper Nyack, NY, 9Global Healthy Living Foundation, Nyack, NY, 10University of Alabama at Birmingham, Hoover, AL, 11Berkshire Medical Center, Lenox, MA
Background/Purpose: Only 37% of patients with rheumatoid arthritis (RA) receive screening for cardiovascular disease (CVD) despite their high risk. To improve CVD prevention, we designed the CArdiovascular Risk assEssment for patients with Rheumatoid Arthritis (CARE RA) intervention. It consists of patient education alongside the assistance of a peer coach. A peer coach is a person with RA that has been trained to coach another person with RA to request a CVD risk assessment from their rheumatologist. The goal of this abstract is to present the preliminary results for this intervention.
Methods: This is a randomized controlled trial for patients with RA. We included patients with RA between 40-75 years of age, no history of CVD, and no cholesterol test or CVD risk assessment in the past 2 years. We excluded people with diabetes or already on lipid lowering therapy (LLT). Participants were randomized to either CARE RA or a control group. CARE RA participants completed 4 weekly calls with a peer coach right before their rheumatologist appointment, to coach their client on requesting CVD risk from their rheumatologist, and one call the week after their appointment to discuss steps to follow once the intervention was completed. The calls followed a curriculum on RA and CVD, that included strategies to lower the risk for CVD and the importance of a CVD risk assessment. The control arm completed the same curriculum but without the assistance of a peer coach. The primary outcome was the number of participants who received a CVD risk assessment defined as, having a cholesterol test, discussing the results of a cholesterol test with their rheumatologist, or initiating LLT if indicated. Data was collected at baseline (5 weeks prior to the rheumatologist appointment), 1-week, and 3-months after the appointment. Differences between arms were determined at 1-week and 3-months after the rheumatologist appointment.
Results: There were 60 participants (Figure 1); 4 dropped out from CARE RA and 4 from the control arm; mean age was 57, 90% females, 80% white (Table 1). At 1-week, 22 participants in CARE RA versus 18 in the control arm received a CVD risk assessment. At 3-month, all participants that completed CARE RA had a CVD risk assessment (Figure 2; p=0.62) and 5 initiated LLT. No participant in the control arm initiated LLT.
Conclusion: The CARE RA intervention seems feasible by means of participants completing the intervention with the peer coaches. Preliminarily, CARE RA seems promote CVD risk assessment, but a larger sample is needed to obtain definitive results about its effectiveness.
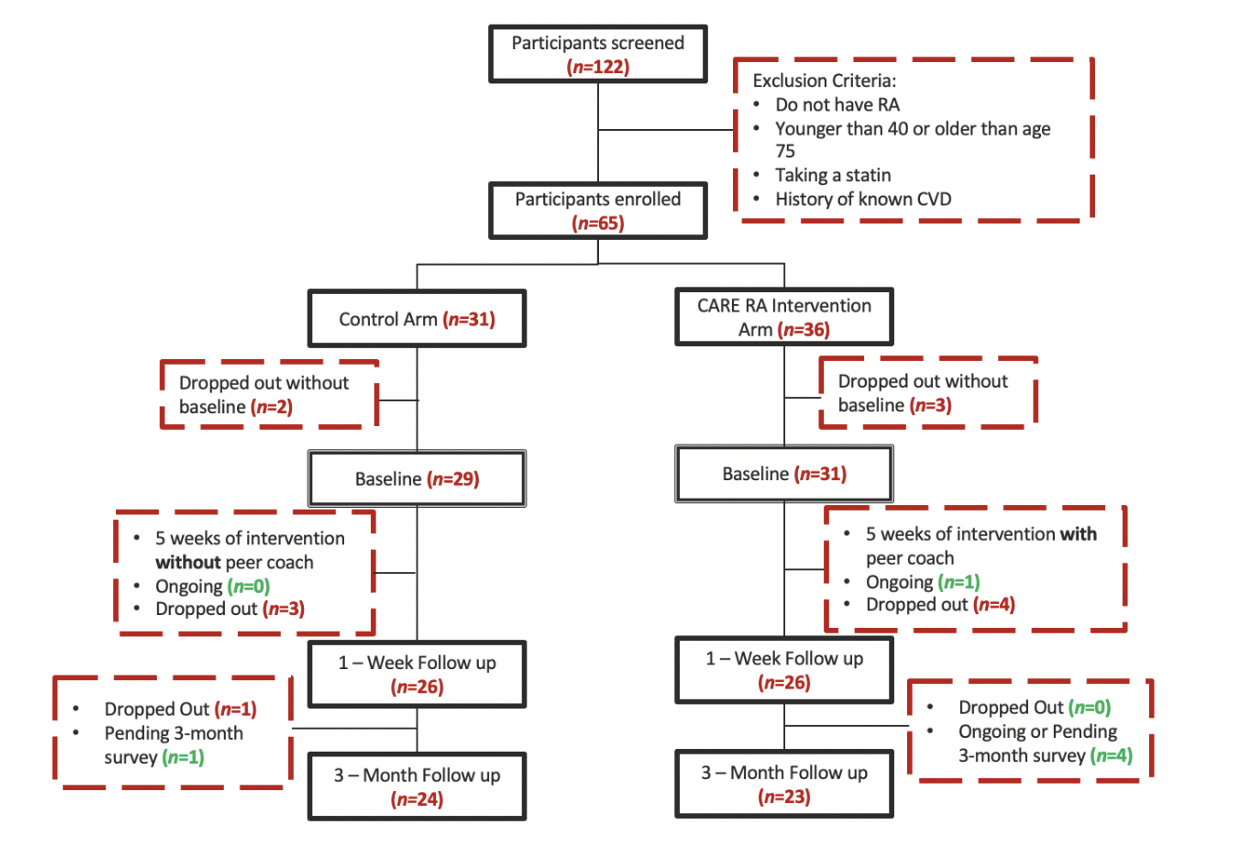 Figure 1. Flow diagram of the patients screened and recruited in the pilot of the CARE RA intervention
Figure 1. Flow diagram of the patients screened and recruited in the pilot of the CARE RA intervention
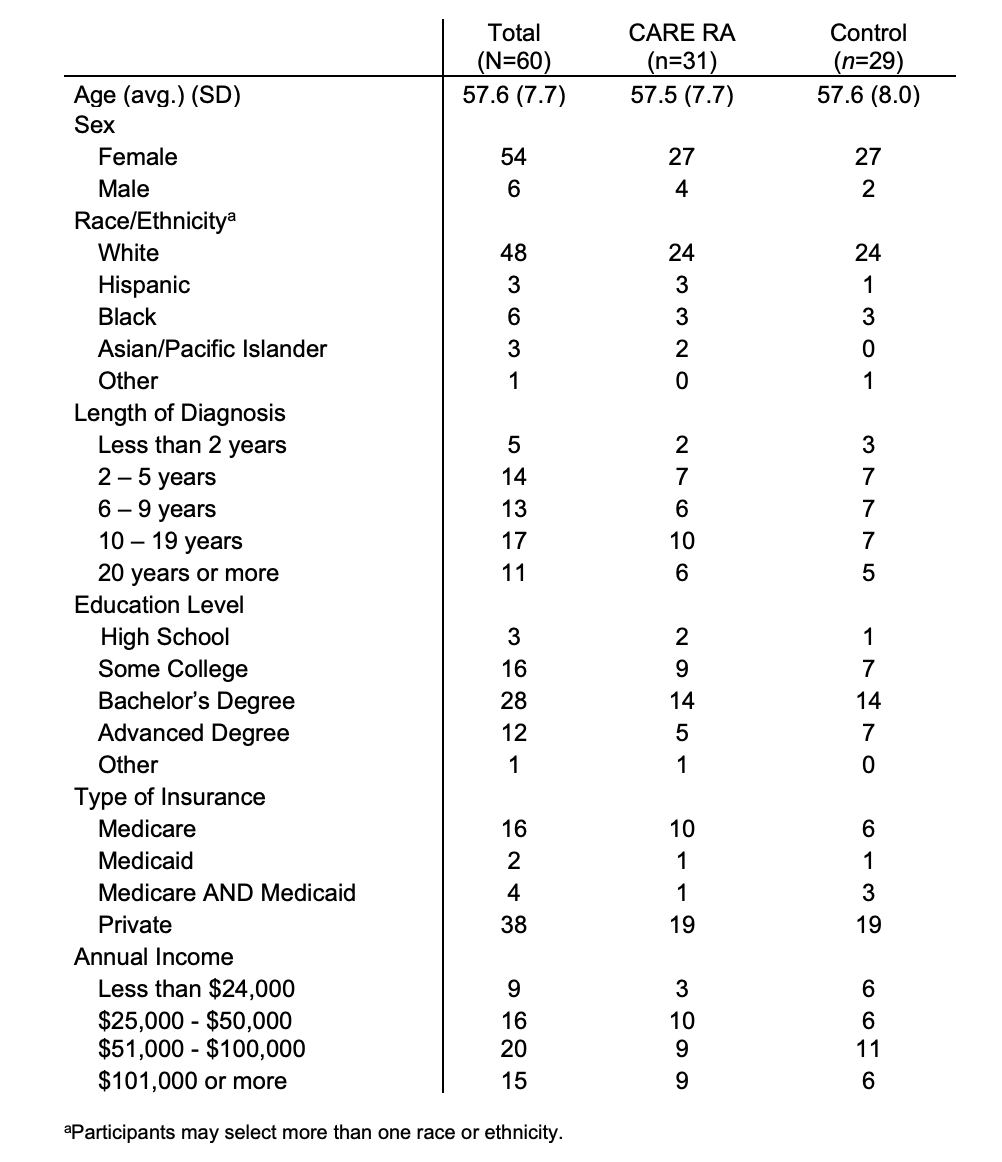 Table 1. Baseline Demographics for Participants Enrolled in the CARE RA Program
Table 1. Baseline Demographics for Participants Enrolled in the CARE RA Program
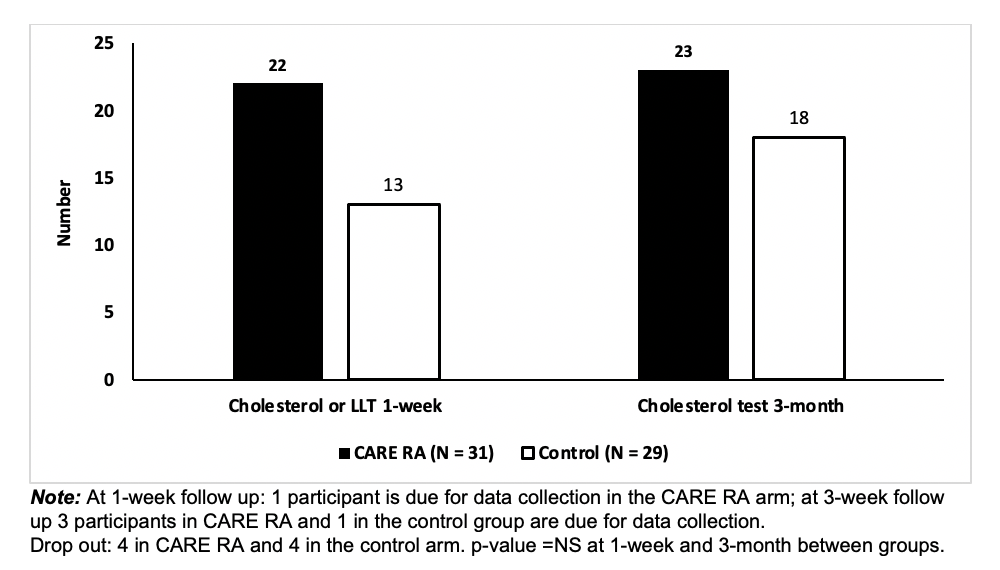 Figure 2. Number of Participants with Cholesterol Test or LLT Initiation at 1-Week and 3-Month After Completion of CARE RA
Figure 2. Number of Participants with Cholesterol Test or LLT Initiation at 1-Week and 3-Month After Completion of CARE RA
Disclosures: I. Navarro-Millan, Sweden Orphan Biovitrum (SOBI); M. Brown, None; Y. Domínguez Páez, None; G. Lui, None; J. Weiner, None; S. fritz, None; T. Sydnor-Campbell, None; A. Allen, None; A. Jabri, None; S. Venkatachalam, None; K. Gavigan, None; W. Nowell, Global Healthy Living Foundation, AbbVie Inc., Amgen, Eli Lilly; J. Curtis, AbbVie/Abbott, Amgen, ArthritisPower, Aqtual, Bendcare, Bristol-Myers Squibb(BMS), CorEvitas, FASTER, GlaxoSmithKlein(GSK), IlluminationHealth, Janssen, Labcorp, Eli Lilly, Myriad, Novartis, Pfizer, Sanofi, Scipher, Setpoint, UCB, United Rheumatology; L. Fraenkel, None; M. Safford, None.
Background/Purpose: Only 37% of patients with rheumatoid arthritis (RA) receive screening for cardiovascular disease (CVD) despite their high risk. To improve CVD prevention, we designed the CArdiovascular Risk assEssment for patients with Rheumatoid Arthritis (CARE RA) intervention. It consists of patient education alongside the assistance of a peer coach. A peer coach is a person with RA that has been trained to coach another person with RA to request a CVD risk assessment from their rheumatologist. The goal of this abstract is to present the preliminary results for this intervention.
Methods: This is a randomized controlled trial for patients with RA. We included patients with RA between 40-75 years of age, no history of CVD, and no cholesterol test or CVD risk assessment in the past 2 years. We excluded people with diabetes or already on lipid lowering therapy (LLT). Participants were randomized to either CARE RA or a control group. CARE RA participants completed 4 weekly calls with a peer coach right before their rheumatologist appointment, to coach their client on requesting CVD risk from their rheumatologist, and one call the week after their appointment to discuss steps to follow once the intervention was completed. The calls followed a curriculum on RA and CVD, that included strategies to lower the risk for CVD and the importance of a CVD risk assessment. The control arm completed the same curriculum but without the assistance of a peer coach. The primary outcome was the number of participants who received a CVD risk assessment defined as, having a cholesterol test, discussing the results of a cholesterol test with their rheumatologist, or initiating LLT if indicated. Data was collected at baseline (5 weeks prior to the rheumatologist appointment), 1-week, and 3-months after the appointment. Differences between arms were determined at 1-week and 3-months after the rheumatologist appointment.
Results: There were 60 participants (Figure 1); 4 dropped out from CARE RA and 4 from the control arm; mean age was 57, 90% females, 80% white (Table 1). At 1-week, 22 participants in CARE RA versus 18 in the control arm received a CVD risk assessment. At 3-month, all participants that completed CARE RA had a CVD risk assessment (Figure 2; p=0.62) and 5 initiated LLT. No participant in the control arm initiated LLT.
Conclusion: The CARE RA intervention seems feasible by means of participants completing the intervention with the peer coaches. Preliminarily, CARE RA seems promote CVD risk assessment, but a larger sample is needed to obtain definitive results about its effectiveness.
 Figure 1. Flow diagram of the patients screened and recruited in the pilot of the CARE RA intervention
Figure 1. Flow diagram of the patients screened and recruited in the pilot of the CARE RA intervention  Table 1. Baseline Demographics for Participants Enrolled in the CARE RA Program
Table 1. Baseline Demographics for Participants Enrolled in the CARE RA Program Figure 2. Number of Participants with Cholesterol Test or LLT Initiation at 1-Week and 3-Month After Completion of CARE RA
Figure 2. Number of Participants with Cholesterol Test or LLT Initiation at 1-Week and 3-Month After Completion of CARE RADisclosures: I. Navarro-Millan, Sweden Orphan Biovitrum (SOBI); M. Brown, None; Y. Domínguez Páez, None; G. Lui, None; J. Weiner, None; S. fritz, None; T. Sydnor-Campbell, None; A. Allen, None; A. Jabri, None; S. Venkatachalam, None; K. Gavigan, None; W. Nowell, Global Healthy Living Foundation, AbbVie Inc., Amgen, Eli Lilly; J. Curtis, AbbVie/Abbott, Amgen, ArthritisPower, Aqtual, Bendcare, Bristol-Myers Squibb(BMS), CorEvitas, FASTER, GlaxoSmithKlein(GSK), IlluminationHealth, Janssen, Labcorp, Eli Lilly, Myriad, Novartis, Pfizer, Sanofi, Scipher, Setpoint, UCB, United Rheumatology; L. Fraenkel, None; M. Safford, None.

