Back
Abstract Session
Session: Abstracts: Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes II: Imaging (2254–2259)
2254: Detection of Active Inflammatory and Structural Changes Indicative of Axial Spondyloarthritis on MRI of Sacroiliac Joint Using a Deep Learning Framework
Monday, November 14, 2022
11:00 AM – 11:10 AM Eastern Time
Location: Room 204
.png)
Denis Poddubnyy, MD
Charité-Universitätsmedizin Berlin
Berlin, Germany
Presenting Author(s)
Keno Kyrill Bressem1, Lisa Adams1, Fabian Proft2, Kay-Geert Hermann3, Torsten Diekhoff1, Laura Spiller1, Stefan Niehues1, Marcus Makowski4, Bernd Hamm1, Mikhail Protopopov5, valeria Rios-Rodriguez6, Hildrun Haibel7, Judith Rademacher5, Murat Torgutalp7, Robert G Lambert8, Xenofon Baraliakos9, Walter P Maksymowych10, Janis Vahldiek1 and Denis Poddubnyy2, 1Charité – Universitätsmedizin Berlin, Berlin, Germany, 2Department of Gastroenterology, Infectious Diseases and Rheumatology, Charité – Universitätsmedizin Berlin, Berlin, Germany, 3Charité - Universitätsmedizin Berlin, Berlin, Germany, 4Technical University of Munich, Munich, Germany, 5Charité Universitätsmedizin Berlin, Berlin, Germany, 6Charité-Universitätsmedizin Berlin, Berlin, Germany, 7Charité - Universitätsmedizin, Berlin, Berlin, Germany, 8University of Alberta, Edmonton, AB, Canada, 9Rheumazentrum Ruhrgebiet Herne, Herne, Germany, 10Department of Medicine, University of Alberta, Edmonton, AB, Canada
Background/Purpose: Magnetic resonance tomography (MRI) plays a key role in the early diagnosis of axial spondyloarthritis (axSpA). However, the detection of changes indicative of axSpA requires specific expertise, which poses a challenge to non-specialized centers. Deep learning (an advanced machine learning method) based on training an artificial neural network may facilitate and support imaging interpretation in clinical practice.
The purpose of the study was to create a reliable deep learning framework for the detection of active inflammatory and structural changes indicative of axSpA on MRI of sacroiliac joints.
Methods: In this study, MRIs of sacroiliac joints from 477 patients from four cohorts (GESPIC-AS, GESPIC-Crohn, GESPIC-Uveitis and OptiRef comprising 266 patients with and 211 without axSpA) were used to develop a deep learning framework (randomly divided into training, n=404, and validation, n=73, datasets). MRIs from the ASAS cohort (n=116) were used for independent testing (test dataset). Each examination in the training/validation dataset was evaluated for the presence of active inflammatory and structural changes indicative of SpA by six experienced, trained and calibrated readers and by seven expert readers in the test dataset. The presence of the changes was defined as the majority vote amongst readers. Discordant cases in the training/validation dataset underwent consensus reading. In addition, the test dataset was evaluated by three radiologists not specifically trained in SpA. Diagnostic performance was evaluated using the area under the receiver operating characteristic curve (AUC), accuracy, sensitivity and specificity.
Results: The prevalence of positive imaging findings for active inflammatory/structural changes indicative of axSpA was 41%/51% in the training/validation dataset and 22%/22% in the test dataset. The model for the detection of active inflammatory changes showed an AUC of 0.91 (0.83 – 0.97) – Figure 1 – and an accuracy of 84% on the validation dataset; the corresponding sensitivity and specificity were 96% and 76%, respectively. Despite a substantially lower prevalence of active inflammatory changes in the test dataset, the model showed good generalization with an AUC of 0.91 (0.84−0.97) and an accuracy of 75%; the sensitivity and specificity were 88% and 71%, respectively. The model demonstrated a similar performance on the validation and test datasets for the detection of active inflammatory changes fulfilling the ASAS definition. The model for the detection of structural changes indicative of axSpA showed good performance on the validation dataset with an AUC of 0.90 (0.82-0.96) for the detection of structural changes and an overall accuracy of 85%. The associated sensitivity and specificity were 95% and 75%, respectively. The model showed reasonable generalization to new data with an AUC of 0.89 (0.81−0.96) and an accuracy of 79%; the sensitivity and specificity were 85% and 78%, respectively. Overall, the model performed close to the individual human experts - Figure 1.
Conclusion: The developed framework allowed the detection of active inflammatory and structural changes indicative of axSpA on MRI. This approach may be used as an assistant tool in the diagnostic workflow.
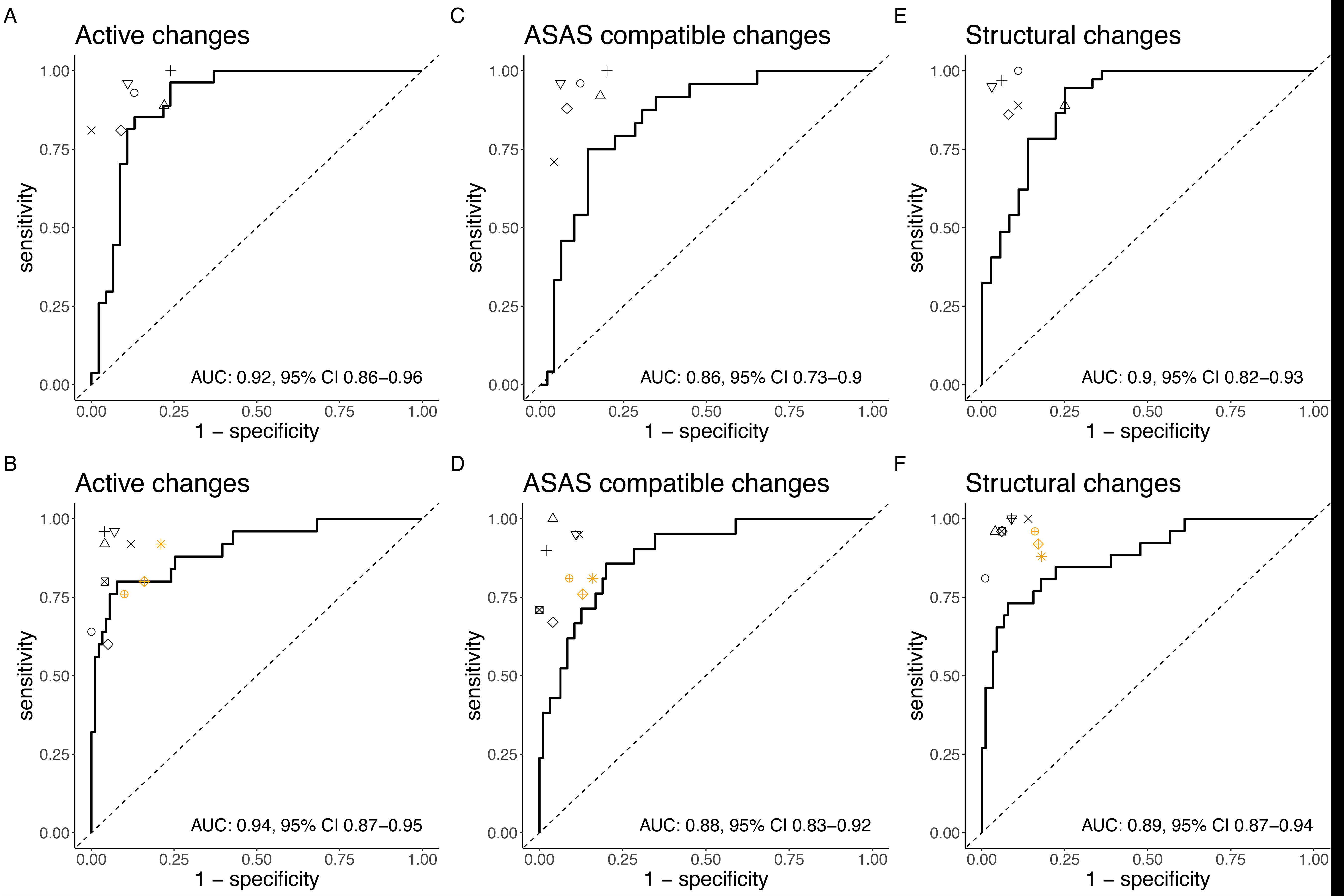 Figure 1. ROC curves and associated AUCs for model performance compared to the individual human experts.
Figure 1. ROC curves and associated AUCs for model performance compared to the individual human experts.
Disclosures: K. Bressem, None; L. Adams, None; F. Proft, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Merck/MSD, Novartis, Pfizer, Roche, UCB; K. Hermann, AbbVie, Merck/MSD, Pfizer, Novartis, BerlinFlame GmbH; T. Diekhoff, Novartis, Merck/MSD, Canon MS, Eli Lilly; L. Spiller, None; S. Niehues, None; M. Makowski, None; B. Hamm, None; M. Protopopov, None; v. Rios-Rodriguez, Falk, AbbVie/Abbott; H. Haibel, Boehringer-Ingelheim, Janssen, Merck/MSD, Novartis, Roche, Sobi, Pfizer, AbbVie/Abbott; J. Rademacher, Novartis, UCB; M. Torgutalp, UCB, AbbVie/Abbott; R. Lambert, Calyx, CARE Arthritis, Image Analysis Group; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited; J. Vahldiek, None; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB.
Background/Purpose: Magnetic resonance tomography (MRI) plays a key role in the early diagnosis of axial spondyloarthritis (axSpA). However, the detection of changes indicative of axSpA requires specific expertise, which poses a challenge to non-specialized centers. Deep learning (an advanced machine learning method) based on training an artificial neural network may facilitate and support imaging interpretation in clinical practice.
The purpose of the study was to create a reliable deep learning framework for the detection of active inflammatory and structural changes indicative of axSpA on MRI of sacroiliac joints.
Methods: In this study, MRIs of sacroiliac joints from 477 patients from four cohorts (GESPIC-AS, GESPIC-Crohn, GESPIC-Uveitis and OptiRef comprising 266 patients with and 211 without axSpA) were used to develop a deep learning framework (randomly divided into training, n=404, and validation, n=73, datasets). MRIs from the ASAS cohort (n=116) were used for independent testing (test dataset). Each examination in the training/validation dataset was evaluated for the presence of active inflammatory and structural changes indicative of SpA by six experienced, trained and calibrated readers and by seven expert readers in the test dataset. The presence of the changes was defined as the majority vote amongst readers. Discordant cases in the training/validation dataset underwent consensus reading. In addition, the test dataset was evaluated by three radiologists not specifically trained in SpA. Diagnostic performance was evaluated using the area under the receiver operating characteristic curve (AUC), accuracy, sensitivity and specificity.
Results: The prevalence of positive imaging findings for active inflammatory/structural changes indicative of axSpA was 41%/51% in the training/validation dataset and 22%/22% in the test dataset. The model for the detection of active inflammatory changes showed an AUC of 0.91 (0.83 – 0.97) – Figure 1 – and an accuracy of 84% on the validation dataset; the corresponding sensitivity and specificity were 96% and 76%, respectively. Despite a substantially lower prevalence of active inflammatory changes in the test dataset, the model showed good generalization with an AUC of 0.91 (0.84−0.97) and an accuracy of 75%; the sensitivity and specificity were 88% and 71%, respectively. The model demonstrated a similar performance on the validation and test datasets for the detection of active inflammatory changes fulfilling the ASAS definition. The model for the detection of structural changes indicative of axSpA showed good performance on the validation dataset with an AUC of 0.90 (0.82-0.96) for the detection of structural changes and an overall accuracy of 85%. The associated sensitivity and specificity were 95% and 75%, respectively. The model showed reasonable generalization to new data with an AUC of 0.89 (0.81−0.96) and an accuracy of 79%; the sensitivity and specificity were 85% and 78%, respectively. Overall, the model performed close to the individual human experts - Figure 1.
Conclusion: The developed framework allowed the detection of active inflammatory and structural changes indicative of axSpA on MRI. This approach may be used as an assistant tool in the diagnostic workflow.
 Figure 1. ROC curves and associated AUCs for model performance compared to the individual human experts.
Figure 1. ROC curves and associated AUCs for model performance compared to the individual human experts.Disclosures: K. Bressem, None; L. Adams, None; F. Proft, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Merck/MSD, Novartis, Pfizer, Roche, UCB; K. Hermann, AbbVie, Merck/MSD, Pfizer, Novartis, BerlinFlame GmbH; T. Diekhoff, Novartis, Merck/MSD, Canon MS, Eli Lilly; L. Spiller, None; S. Niehues, None; M. Makowski, None; B. Hamm, None; M. Protopopov, None; v. Rios-Rodriguez, Falk, AbbVie/Abbott; H. Haibel, Boehringer-Ingelheim, Janssen, Merck/MSD, Novartis, Roche, Sobi, Pfizer, AbbVie/Abbott; J. Rademacher, Novartis, UCB; M. Torgutalp, UCB, AbbVie/Abbott; R. Lambert, Calyx, CARE Arthritis, Image Analysis Group; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited; J. Vahldiek, None; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB.

