Back
Poster Session B
Infection-related rheumatic syndromes
Session: (0779–0806) Infection-related Rheumatic Disease Poster
0795: Post-Acute COVID-19 Sequalae (PACS) with New-onset Rheumatological Complications
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- MM
Manali Mukherjee, PhD
McMaster University
Hamilton, ON, Canada
Abstract Poster Presenter(s)
Neel Thanavala1, Kiho Son2, Nardien Sedhom2, Snehal Somalwar3, Zil Patel1, Rameen Jamil2, Carmen Venegas2, Ashutosh Thakar2, Agnes Yuen4, Melanie Kjarsgaard2, Susan Waserman2, Mylinh Duong2, Parameswaran Nair2, Christopher Carlsten4, Maggie Larche2, Terence Ho2, Sarah Svenningsen2, KONSTANTINOS TSELIOS5 and Manali Mukherjee2, 1The Research Institute of St. Joe's Hamilton, Hamilton, ON, Canada, 2McMaster University, Hamilton, ON, Canada, 3The Research Institute of St Joe's Hamilton, Hamilton, ON, Canada, 4University of British Columbia, Vancouver, BC, Canada, 5McMaster University, Population Health Research Institute, Hamilton, ON, Canada
Background/Purpose: Post-Acute Sequalae of COVID-19 (PASC), prevalent in ~20-30% of convalescent COVID-19 patients is characterized by new/persistent symptoms after the initial recovery. Certain autoantibodies have been described in acute COVID-19 but their clinical significance is unknown. The purpose of this study was the assessment of such antibodies in PASC, their association with symptom persistence as well as the potential for the development of future rheumatic and/or autoimmune diseases.
Methods: In a multi-center, observational study (Post-COVID), we enrolled n=58 PCR+ COVID-19 patients between August'20-September'21 +/- symptoms, at 12 months post-recovery. In a subsequent, observational study (Autoimmunity in Post-Acute COVID Sequelae, AIPACS), between September'21-April'22, we recruited n=58 PASC patients (persistent symptoms for >12 weeks, PCR-confirmed) at 3-6 months (n=11), 6-9 months (n=9), 9-12 months (n=18), and >12 months (n=20) post-infection. All patients were ≥18 years, without history of autoimmune disease. Rapid assessment line immunoassays (HUMAN Diagnostics, Germany) were used to detect circulating autoantibodies.
Results: In the Post-COVID study, 43% (25/58) of patients reported symptoms (fatigue, dyspnea, or cough) at 12 months post-infection. Anti-U1-snRNP (30%) and anti-SS-B/La (21%) were the most prevalent autoantibodies (Fig. 1A) that positively correlated with and predicted persisting symptoms of fatigue and dyspnea at 12-month post-infection (simple logistic regression, P< 0.05) (Fig. 1B, C). In the AIPACS study, patients at >12 months post-infection had significantly higher numbers of positive autoreactivities compared to patients 6-12 months post-infection (p=0.03) (Fig. 1D). Finally, six patients (10%, 2 males/4 females, median age 42 years) were newly diagnosed with an autoimmune and/or rheumatic disease; systemic lupus erythematosus (n=1), postural orthostatic tachycardia syndrome (n=3), psoriasis (n=1) and polymyalgia rheumatica (n=1) (Table 1). Anti-SS-B/La and/or anti-U1-snRNP were detectable in 5/6 patients.
Conclusion: Autoantibodies to certain nuclear antigens were detected in PASC patients beyond 12 months post-infection. A subset of PASC patients without prior history of autoimmunity may be susceptible to new-onset rheumatological complications.
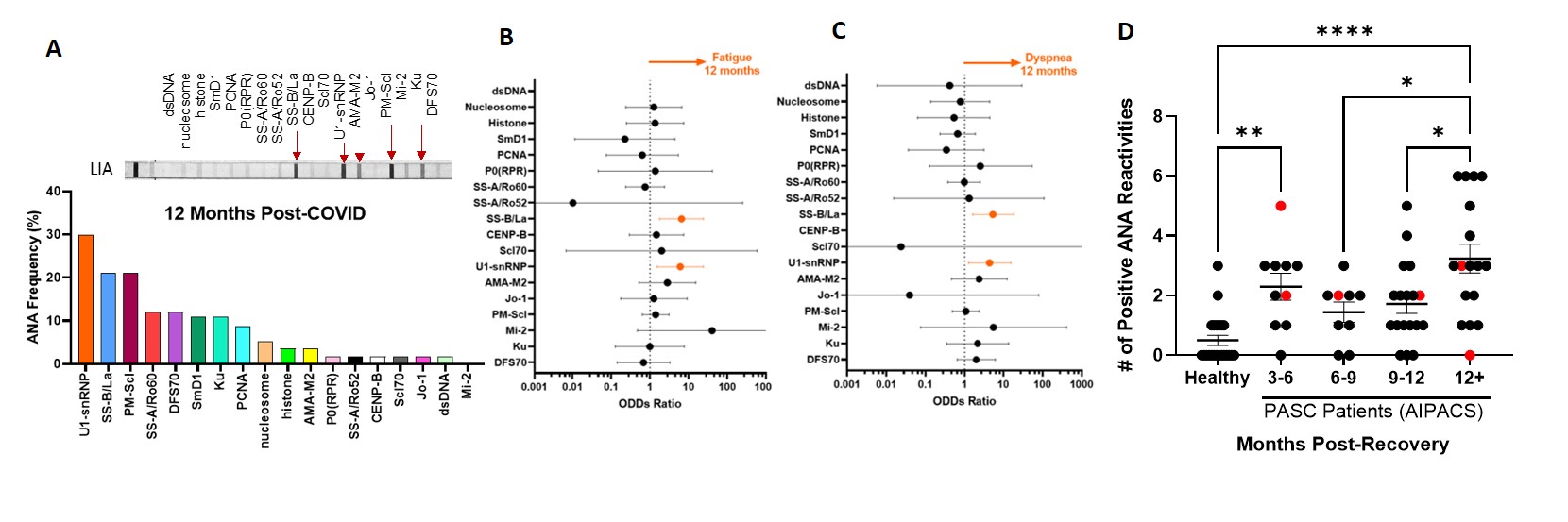 Figure 1. Prevalence of circulating anti-nuclear/extractable nuclear antibodies: Histograms visualizing the most prevalent ANAs at 12 months post-infection time point of 58 convalescent covid-19 patients with a representative line immunoassay strip showing positive band intensities (A). Regression analysis showing the ODDs ratio for predicting fatigue and dyspnea persistent at 12 months (B,C) in Post-COVID study. Scatter plot showing the number of ANA reactivities per patient in AIPAC study (n=58) as a cross-sectional dataset- over the different recovery time frames, compared to an age-sex matched healthy never-COVID pre-vaccinated healthy control (n=22). Red symbols indicate the seven patients diagnosed with an autoimmune disease during AIPAC study visit (rheumatology consult). Kruskal Wallis with multiple comparison test.
Figure 1. Prevalence of circulating anti-nuclear/extractable nuclear antibodies: Histograms visualizing the most prevalent ANAs at 12 months post-infection time point of 58 convalescent covid-19 patients with a representative line immunoassay strip showing positive band intensities (A). Regression analysis showing the ODDs ratio for predicting fatigue and dyspnea persistent at 12 months (B,C) in Post-COVID study. Scatter plot showing the number of ANA reactivities per patient in AIPAC study (n=58) as a cross-sectional dataset- over the different recovery time frames, compared to an age-sex matched healthy never-COVID pre-vaccinated healthy control (n=22). Red symbols indicate the seven patients diagnosed with an autoimmune disease during AIPAC study visit (rheumatology consult). Kruskal Wallis with multiple comparison test.
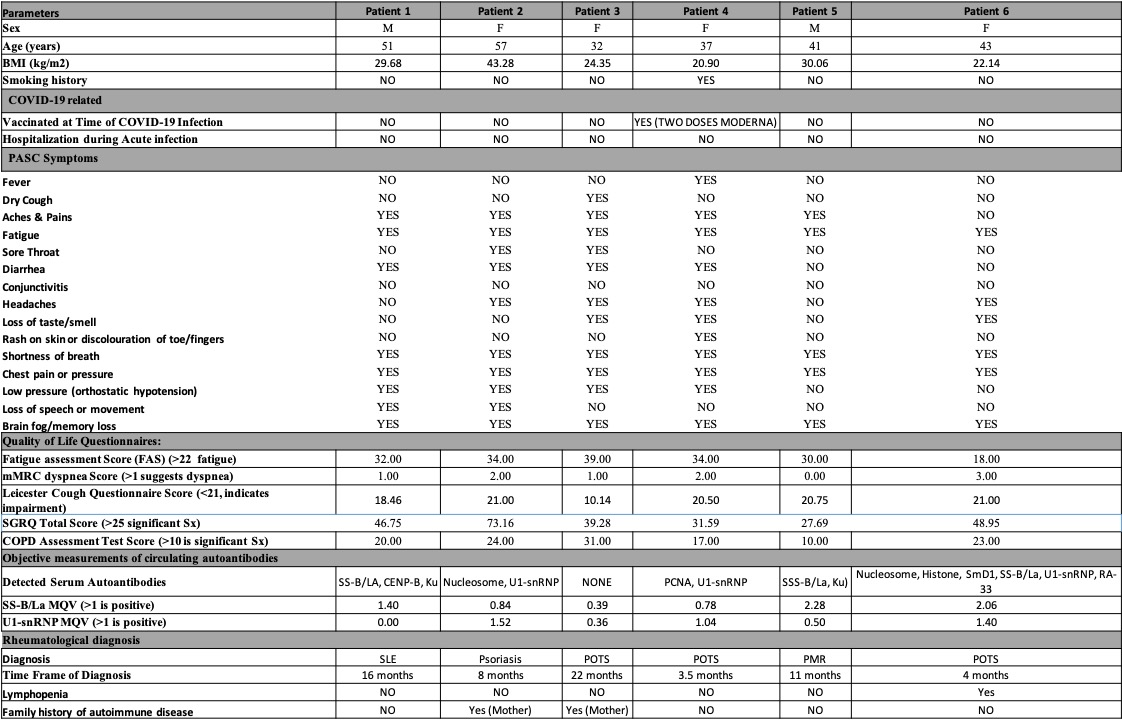 N.B. BMI - Body mass index; MQV is mean quantitative value of the band intensity on the ANA Line immunoassay; Sx - symptoms; mMRC - modified Medical Research Council; SGRQ - St George's Respiratory Questionnaire; ANA - antinuclear/extractable nuclear antibodies; SLE - systemic lupus erythematosus; POTS - postural orthostatic tachycardia syndrome; PMR - polymyalgia rheumatica;
N.B. BMI - Body mass index; MQV is mean quantitative value of the band intensity on the ANA Line immunoassay; Sx - symptoms; mMRC - modified Medical Research Council; SGRQ - St George's Respiratory Questionnaire; ANA - antinuclear/extractable nuclear antibodies; SLE - systemic lupus erythematosus; POTS - postural orthostatic tachycardia syndrome; PMR - polymyalgia rheumatica;
Disclosures: N. Thanavala, None; K. Son, None; N. Sedhom, None; S. Somalwar, None; Z. Patel, None; R. Jamil, None; C. Venegas, None; A. Thakar, None; A. Yuen, None; M. Kjarsgaard, None; S. Waserman, Alk Abello, CAAIF, Aimmune, Takeda, Siolta, GlaxoSmithKlein(GSK), AstraZeneca, Novartis, CSL Behring, Pfizer, Sanofi, Medexus, Miravo Health, AbbVie, Bausch Lomb; M. Duong, None; P. Nair, AstraZeneca, Teva, Sanofi, Equillium, Foresee, Arrowhead Pharma, Cyclomedica, GlaxoSmithKlein(GSK); C. Carlsten, None; M. Larche, None; T. Ho, Fisher and Paykel, Sanofi, Valeo, AstraZeneca; S. Svenningsen, Cyclomedica, Arrowhead Pharmaceuticals, AstraZeneca, Novartis, Polarean; K. TSELIOS, AstraZeneca, GlaxoSmithKlein(GSK); M. Mukherjee, COVID-19 Immunity Task Force (CIHR), Methapharm Specialty Pharmaceuticals, Canadian Asthma Allergy Immunology Foundation, Canadian Institutes of Health Research, Novartis, GlaxoSmithKline, AstraZeneca.
Background/Purpose: Post-Acute Sequalae of COVID-19 (PASC), prevalent in ~20-30% of convalescent COVID-19 patients is characterized by new/persistent symptoms after the initial recovery. Certain autoantibodies have been described in acute COVID-19 but their clinical significance is unknown. The purpose of this study was the assessment of such antibodies in PASC, their association with symptom persistence as well as the potential for the development of future rheumatic and/or autoimmune diseases.
Methods: In a multi-center, observational study (Post-COVID), we enrolled n=58 PCR+ COVID-19 patients between August'20-September'21 +/- symptoms, at 12 months post-recovery. In a subsequent, observational study (Autoimmunity in Post-Acute COVID Sequelae, AIPACS), between September'21-April'22, we recruited n=58 PASC patients (persistent symptoms for >12 weeks, PCR-confirmed) at 3-6 months (n=11), 6-9 months (n=9), 9-12 months (n=18), and >12 months (n=20) post-infection. All patients were ≥18 years, without history of autoimmune disease. Rapid assessment line immunoassays (HUMAN Diagnostics, Germany) were used to detect circulating autoantibodies.
Results: In the Post-COVID study, 43% (25/58) of patients reported symptoms (fatigue, dyspnea, or cough) at 12 months post-infection. Anti-U1-snRNP (30%) and anti-SS-B/La (21%) were the most prevalent autoantibodies (Fig. 1A) that positively correlated with and predicted persisting symptoms of fatigue and dyspnea at 12-month post-infection (simple logistic regression, P< 0.05) (Fig. 1B, C). In the AIPACS study, patients at >12 months post-infection had significantly higher numbers of positive autoreactivities compared to patients 6-12 months post-infection (p=0.03) (Fig. 1D). Finally, six patients (10%, 2 males/4 females, median age 42 years) were newly diagnosed with an autoimmune and/or rheumatic disease; systemic lupus erythematosus (n=1), postural orthostatic tachycardia syndrome (n=3), psoriasis (n=1) and polymyalgia rheumatica (n=1) (Table 1). Anti-SS-B/La and/or anti-U1-snRNP were detectable in 5/6 patients.
Conclusion: Autoantibodies to certain nuclear antigens were detected in PASC patients beyond 12 months post-infection. A subset of PASC patients without prior history of autoimmunity may be susceptible to new-onset rheumatological complications.
 Figure 1. Prevalence of circulating anti-nuclear/extractable nuclear antibodies: Histograms visualizing the most prevalent ANAs at 12 months post-infection time point of 58 convalescent covid-19 patients with a representative line immunoassay strip showing positive band intensities (A). Regression analysis showing the ODDs ratio for predicting fatigue and dyspnea persistent at 12 months (B,C) in Post-COVID study. Scatter plot showing the number of ANA reactivities per patient in AIPAC study (n=58) as a cross-sectional dataset- over the different recovery time frames, compared to an age-sex matched healthy never-COVID pre-vaccinated healthy control (n=22). Red symbols indicate the seven patients diagnosed with an autoimmune disease during AIPAC study visit (rheumatology consult). Kruskal Wallis with multiple comparison test.
Figure 1. Prevalence of circulating anti-nuclear/extractable nuclear antibodies: Histograms visualizing the most prevalent ANAs at 12 months post-infection time point of 58 convalescent covid-19 patients with a representative line immunoassay strip showing positive band intensities (A). Regression analysis showing the ODDs ratio for predicting fatigue and dyspnea persistent at 12 months (B,C) in Post-COVID study. Scatter plot showing the number of ANA reactivities per patient in AIPAC study (n=58) as a cross-sectional dataset- over the different recovery time frames, compared to an age-sex matched healthy never-COVID pre-vaccinated healthy control (n=22). Red symbols indicate the seven patients diagnosed with an autoimmune disease during AIPAC study visit (rheumatology consult). Kruskal Wallis with multiple comparison test. N.B. BMI - Body mass index; MQV is mean quantitative value of the band intensity on the ANA Line immunoassay; Sx - symptoms; mMRC - modified Medical Research Council; SGRQ - St George's Respiratory Questionnaire; ANA - antinuclear/extractable nuclear antibodies; SLE - systemic lupus erythematosus; POTS - postural orthostatic tachycardia syndrome; PMR - polymyalgia rheumatica;
N.B. BMI - Body mass index; MQV is mean quantitative value of the band intensity on the ANA Line immunoassay; Sx - symptoms; mMRC - modified Medical Research Council; SGRQ - St George's Respiratory Questionnaire; ANA - antinuclear/extractable nuclear antibodies; SLE - systemic lupus erythematosus; POTS - postural orthostatic tachycardia syndrome; PMR - polymyalgia rheumatica; Disclosures: N. Thanavala, None; K. Son, None; N. Sedhom, None; S. Somalwar, None; Z. Patel, None; R. Jamil, None; C. Venegas, None; A. Thakar, None; A. Yuen, None; M. Kjarsgaard, None; S. Waserman, Alk Abello, CAAIF, Aimmune, Takeda, Siolta, GlaxoSmithKlein(GSK), AstraZeneca, Novartis, CSL Behring, Pfizer, Sanofi, Medexus, Miravo Health, AbbVie, Bausch Lomb; M. Duong, None; P. Nair, AstraZeneca, Teva, Sanofi, Equillium, Foresee, Arrowhead Pharma, Cyclomedica, GlaxoSmithKlein(GSK); C. Carlsten, None; M. Larche, None; T. Ho, Fisher and Paykel, Sanofi, Valeo, AstraZeneca; S. Svenningsen, Cyclomedica, Arrowhead Pharmaceuticals, AstraZeneca, Novartis, Polarean; K. TSELIOS, AstraZeneca, GlaxoSmithKlein(GSK); M. Mukherjee, COVID-19 Immunity Task Force (CIHR), Methapharm Specialty Pharmaceuticals, Canadian Asthma Allergy Immunology Foundation, Canadian Institutes of Health Research, Novartis, GlaxoSmithKline, AstraZeneca.

