Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0883–0912) RA – Diagnosis, Manifestations, and Outcomes Poster II
0892: Predicting Value of Circulating Semaphorin 4A for Rheumatoid Arthritis Progression and Response to Treatment
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

Jérôme Avouac, MD, PhD
Université de Paris Cité
Paris, France
Abstract Poster Presenter(s)
Jérôme Avouac1, Eloïse Vandebeuque2, Alice Combier3, lucile poiroux3, Alexia Steelandt3, Margaux Boisson3, Virginie Gonzalez4, Anne Cauvet4, Thomas Barnetche5, Marie Truchetet6, Christophe Richez7 and Yannick Allanore8, 1University of Paris, Paris, France, 2hôpital Cochin, AP-HP AP-HP.Centre - Université Paris Cité, Paris, France, 3Hôpital Cochin, AP-HP.Centre - Université Paris Cité, Paris, France, 4INSERM U1016, Institut Cochin, Paris, France, 5CHU Bordeaux, Bordeaux, France, 6Bordeaux University Hospital, Bordeaux, France, 7Université de Bordeaux, Bordeaux, France, 8Department of Rheumatology A, Descartes University, APHP, Cochin Hospital, Paris, France, Paris, France
Background/Purpose: The lack of validated tools to predict rheumatoid arthritis (RA) disease course warrants the development of new reliable biomarkers. We have previously detected increased semaphorin 4A (SEMA4A) expression in endothelial cells, synovial tissue, and serum of patients with RA. In addition, SEMA4A serum levels correlated with multiple clinical, biological, and power doppler ultrasound markers of disease activity and angiogenesis.
Our objective was to evaluate circulating SEMA4A for the prediction of disease progression in RA and study its association with response to therapy.
Methods: A first cohort from Paris, France, included between May 2016 and February 2018 101 consecutive RA patients followed up on an annual basis until August 2021. Baseline SEMA4A concentrations measured by quantitative ELISAs (Coud-Clone Corp, Katy, TX) were analyzed according to disease progression, defined by the occurrence of patient-reported flares and initiation or change of targeted therapy related to persistent disease activity. Increased SEMA4A concentrations were defined as values >94 ng/mL, as previously reported (Avouac et al, Arthritis Rheumatol 2021). A second cohort from Bordeaux, France, included consecutive RA patients who initiated new therapy because of insufficient disease control. The course of SEMA4A levels was studied between baseline and month 3 according to treatment response.
Results: A total of 101 patients (85 females, 84%) were included in the first cohort, with a mean age of 58±13 years and a mean disease duration of 14±11 years. During a follow-up period of 41±15 months, disease progression occurred in 26 patients.
Increased baseline SEMA4A levels were predictive of disease progression (hazard ratio, HR: 2.73, 95%CI 1.24-5.96, respectively) (Figure 1A). Multivariate Cox analyses confirmed that SEMA4A was an independent predictors of disease progression (HR: 2.71, 95%CI 1.14-6.43, respectively). SEMA4A remained predictive of disease progression in the 58 patients with a DAS28 < 3.2 at baseline (HR: 3.50, 95%CI 1.02-12.01, respectively).
SEM4A was also confirmed as an independent predictor of flares (n=38), together with the DAS28 and synovial hyperhemia. Baseline SEMA4A levels also identified more active and difficult to treat patients who maintained higher mean DAS28-CRP values during the follow-up period (Figure 1B).
A total of 40 patients (29 females, 73%) initiating new therapy (15 methotrexate and 25 tocilizumab) were included in the second cohort. These patients had a mean age of 57±14 years, a mean disease duration of 5±6 years. 4 patients experienced no treatment response, 10 had a moderate response and 26 a good response. SEMA4A concentrations were markedly decreased between m0 and m3 in the group of patients with good clinical response (Figure 1C).
Conclusion: Circulating SEMA4A is a robust biomarker of disease progression, associated with therapy responsiveness.
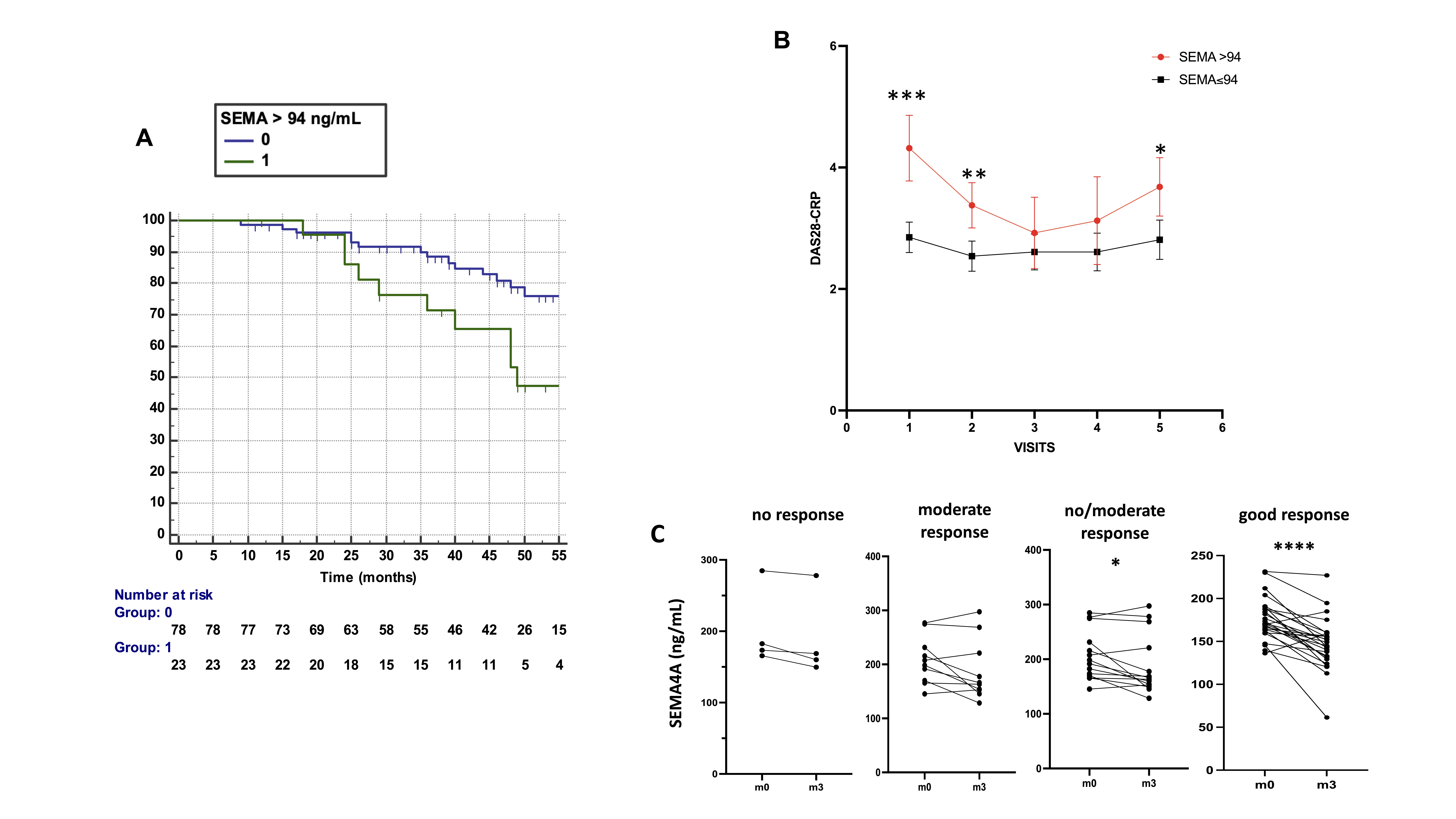 Figure 1A-C: Predictive value of SEMA4A for the progression and response to treatment of rheumatoid arthritis. A, Time to disease progression according to circulating SEMA4A concentrations (≤ or > 94 ng/mL). B, Course of the DAS28 during the follow-up period according to baseline SEMA4A concentrations (≤ or > 94 ng/mL). All data are shown as the mean ± SEM. * p < 0.05, ** p < 0.01 and *** p < 0.001, determined by Student’s t test. C, C-E, Course of SEMA4A concentrations according to response to therapy. * p < 0.05 and ****p < 0.0001, determined by the Wilcoxon matched-pairs signed rank test.
Figure 1A-C: Predictive value of SEMA4A for the progression and response to treatment of rheumatoid arthritis. A, Time to disease progression according to circulating SEMA4A concentrations (≤ or > 94 ng/mL). B, Course of the DAS28 during the follow-up period according to baseline SEMA4A concentrations (≤ or > 94 ng/mL). All data are shown as the mean ± SEM. * p < 0.05, ** p < 0.01 and *** p < 0.001, determined by Student’s t test. C, C-E, Course of SEMA4A concentrations according to response to therapy. * p < 0.05 and ****p < 0.0001, determined by the Wilcoxon matched-pairs signed rank test.
Disclosures: J. Avouac, Galapagos, AbbVie, Lilly, Pfizer, Bristol Myers Squibb, Novartis, Fresenius-Kabi, Sanofi, Sandoz, Nordic Pharma, Biogen, Medac, Janssen, Roche-Chugai; E. Vandebeuque, None; A. Combier, None; l. poiroux, None; A. Steelandt, None; M. Boisson, None; V. Gonzalez, None; A. Cauvet, None; T. Barnetche, None; M. Truchetet, AbbVie/Abbott, Pfizer, Eli Lilly, Galapados; C. Richez, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Galapados, GlaxoSmithKlein(GSK), Eli Lilly, Novartis, Pfizer; Y. Allanore, Boehringer Ingelheim, Sanofi, Janssen, AbbVie/Abbott, Menarini, Curzion, Medsenic, Prometheus, AstraZeneca.
Background/Purpose: The lack of validated tools to predict rheumatoid arthritis (RA) disease course warrants the development of new reliable biomarkers. We have previously detected increased semaphorin 4A (SEMA4A) expression in endothelial cells, synovial tissue, and serum of patients with RA. In addition, SEMA4A serum levels correlated with multiple clinical, biological, and power doppler ultrasound markers of disease activity and angiogenesis.
Our objective was to evaluate circulating SEMA4A for the prediction of disease progression in RA and study its association with response to therapy.
Methods: A first cohort from Paris, France, included between May 2016 and February 2018 101 consecutive RA patients followed up on an annual basis until August 2021. Baseline SEMA4A concentrations measured by quantitative ELISAs (Coud-Clone Corp, Katy, TX) were analyzed according to disease progression, defined by the occurrence of patient-reported flares and initiation or change of targeted therapy related to persistent disease activity. Increased SEMA4A concentrations were defined as values >94 ng/mL, as previously reported (Avouac et al, Arthritis Rheumatol 2021). A second cohort from Bordeaux, France, included consecutive RA patients who initiated new therapy because of insufficient disease control. The course of SEMA4A levels was studied between baseline and month 3 according to treatment response.
Results: A total of 101 patients (85 females, 84%) were included in the first cohort, with a mean age of 58±13 years and a mean disease duration of 14±11 years. During a follow-up period of 41±15 months, disease progression occurred in 26 patients.
Increased baseline SEMA4A levels were predictive of disease progression (hazard ratio, HR: 2.73, 95%CI 1.24-5.96, respectively) (Figure 1A). Multivariate Cox analyses confirmed that SEMA4A was an independent predictors of disease progression (HR: 2.71, 95%CI 1.14-6.43, respectively). SEMA4A remained predictive of disease progression in the 58 patients with a DAS28 < 3.2 at baseline (HR: 3.50, 95%CI 1.02-12.01, respectively).
SEM4A was also confirmed as an independent predictor of flares (n=38), together with the DAS28 and synovial hyperhemia. Baseline SEMA4A levels also identified more active and difficult to treat patients who maintained higher mean DAS28-CRP values during the follow-up period (Figure 1B).
A total of 40 patients (29 females, 73%) initiating new therapy (15 methotrexate and 25 tocilizumab) were included in the second cohort. These patients had a mean age of 57±14 years, a mean disease duration of 5±6 years. 4 patients experienced no treatment response, 10 had a moderate response and 26 a good response. SEMA4A concentrations were markedly decreased between m0 and m3 in the group of patients with good clinical response (Figure 1C).
Conclusion: Circulating SEMA4A is a robust biomarker of disease progression, associated with therapy responsiveness.
 Figure 1A-C: Predictive value of SEMA4A for the progression and response to treatment of rheumatoid arthritis. A, Time to disease progression according to circulating SEMA4A concentrations (≤ or > 94 ng/mL). B, Course of the DAS28 during the follow-up period according to baseline SEMA4A concentrations (≤ or > 94 ng/mL). All data are shown as the mean ± SEM. * p < 0.05, ** p < 0.01 and *** p < 0.001, determined by Student’s t test. C, C-E, Course of SEMA4A concentrations according to response to therapy. * p < 0.05 and ****p < 0.0001, determined by the Wilcoxon matched-pairs signed rank test.
Figure 1A-C: Predictive value of SEMA4A for the progression and response to treatment of rheumatoid arthritis. A, Time to disease progression according to circulating SEMA4A concentrations (≤ or > 94 ng/mL). B, Course of the DAS28 during the follow-up period according to baseline SEMA4A concentrations (≤ or > 94 ng/mL). All data are shown as the mean ± SEM. * p < 0.05, ** p < 0.01 and *** p < 0.001, determined by Student’s t test. C, C-E, Course of SEMA4A concentrations according to response to therapy. * p < 0.05 and ****p < 0.0001, determined by the Wilcoxon matched-pairs signed rank test.Disclosures: J. Avouac, Galapagos, AbbVie, Lilly, Pfizer, Bristol Myers Squibb, Novartis, Fresenius-Kabi, Sanofi, Sandoz, Nordic Pharma, Biogen, Medac, Janssen, Roche-Chugai; E. Vandebeuque, None; A. Combier, None; l. poiroux, None; A. Steelandt, None; M. Boisson, None; V. Gonzalez, None; A. Cauvet, None; T. Barnetche, None; M. Truchetet, AbbVie/Abbott, Pfizer, Eli Lilly, Galapados; C. Richez, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Galapados, GlaxoSmithKlein(GSK), Eli Lilly, Novartis, Pfizer; Y. Allanore, Boehringer Ingelheim, Sanofi, Janssen, AbbVie/Abbott, Menarini, Curzion, Medsenic, Prometheus, AstraZeneca.

