Back
Poster Session B
Juvenile idiopathic arthritis (JIA) and pediatric joint disorders
Session: (0850–0880) Pediatric Rheumatology – Clinical Poster I: JIA
0875: Predictive Biomarkers of Tofacitinib Response and Disease Activity in Juvenile Idiopathic Arthritis Subtypes: A Longitudinal Study
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- EO
Ekemini Ogbu, MD, MSc
Cincinnati Children's Hospital Medical Center
Cincinnati, OH, United States
Abstract Poster Presenter(s)
Ekemini Ogbu1, Sherry Thornton2, Alyssa Sproles2, Alexei Grom3, Sanjeev Dhakal4, Bin Huang5 and Hermine Brunner6, 1Cincinnati Children's Hospital Medical Center, Atlanta, GA, 2Cincinnati Children's Hospital, Cincinnati, OH, 3Divisions of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, 4Cincinnati Children's Hospital Medical Center, Cincinnati, OH, 5Cincinnati Children's Hospital Medical Center, Cinciannati, OH, 6Division of Rheumatology, Cincinnati Children's Hospital Medical Center, University of Cincinnati, Department of Pediatrics, Cincinnati, OH
Background/Purpose: Tofacitinib has been shown to improve disease activity of several subtypes of Juvenile Idiopathic Arthritis (JIA). No known biomarkers to date predict JIA improvement on Tofacitinib. The purpose of this study was to test the usefulness of candidate blood-based biomarkers (CBB) previously associated with status and course of JIA or rheumatoid arthritis for prediction of JIA disease activity and improvement after 18 weeks of tofacitinib use.
Methods: Data and samples were from the phase 3 randomized clinical trial in JIA (NCT02592434). CBB studied were: S100A8/9, S100A12, IL18, SAA, resistin, VEGF, Angiopoietin-1, Angiopoietin-2, MMP8, MMP2, TIMP1, Leptin, CXCL9, soluble (s) IL2R, ICAM-1, sTNFR, IL6, IL23, MCP-1, CCL18, and CCL20. Samples of 166 patients aged 2-< 18 years with the following JIA subtypes were assayed: 1) polyarticular rheumatoid factor (RF) positive, 2) RF negative, 3) extended oligoarticular, 4) juvenile psoriatic arthritis,5) enthesitis-related arthritis , and 6) systemic (SJIA). All patients received tofacitinib from baseline to week 18 (BL-wk18). Outcomes of treatment response were: change in JIA disease activity score (JADAS27-CRP) and JIA-ACR90 level improvement (BL-wk18).
Results: Primary analysis excluded 7 SJIA patients, given the unique mechanism of autoinflammation in SJIA. Table 1 summarizes BL characteristics of the remaining 159 patients. BL median levels of select CBB differed between JIA subtypes (S100A12, IL18, resistin, VEGF, sTNFR, CCL20), as shown in Table 1. At wk18, samples of 139 patients were available for analysis, 50 (36%) achieved ACR90, 49/20/6 or 35%/14%/4% achieved ACR70/50/30. HLA-B27 positivity was associated with a significantly decreased odds of achieving a JIA-ACR90 level response (Table 2). Thus, the remaining analyses on CBB excluded N=21 HLA-B27 positive records. At BL, JADAS27-CRP was significantly correlated with IL6 (r = 0.24, p=0.004), IL18 (r = 0.18; p=0.03), SAA (r = 0.19; p=0.03), and MIG (0.18, p =0.03). Change of S100A12 significantly predicted the change in JADAS27-CRP (p = 0.0002) and in ACR90 [AUC=0.73, 95%CI of 0.64-0.82], after adjustments for BL JADAS27-CRP, JIA subtype, sex, age of diagnosis and disease duration. Similarly, BL-wk18 change in resistin and BL resistin significantly predicted the change in JADAS27-CRP [R-square=0.78] and in ACR_90 [AUC=0.72, 95%CI of 0.63-0.81]. Stepwise logistic selection identified that BL IL18, and decrease in S100A12 and MCP-1 were excellent predictors of JIA-ACR90 response (AUC: 0.78; 95% CI: 0.70-0.87; Figure 1). Secondary analysis including SJIA patients yielded similar findings. Background methotrexate and/or corticosteroids were not associated with CBB levels at BL or wk18.
Conclusion: While many previously proposed CBB did not predict response of JIA patients to tofacitinib, our exploratory analysis suggests that S100A12, MCP-1 may be predictive of or associated with JIA-ACR90 level improvement and improvement of JIA disease activity at wk18. Further, our analysis suggests that HLA B27 positivity may be associated with lower response to tofacitinib however numbers were small. Confirmation of these findings in an independent validation cohort is needed.
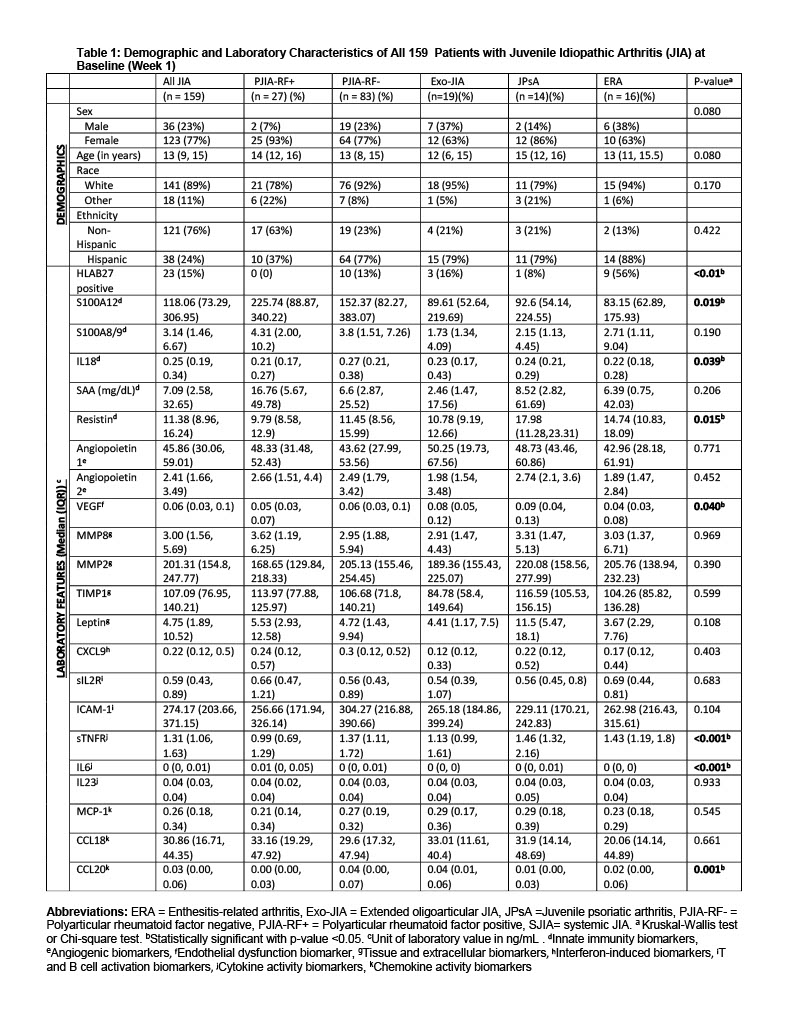
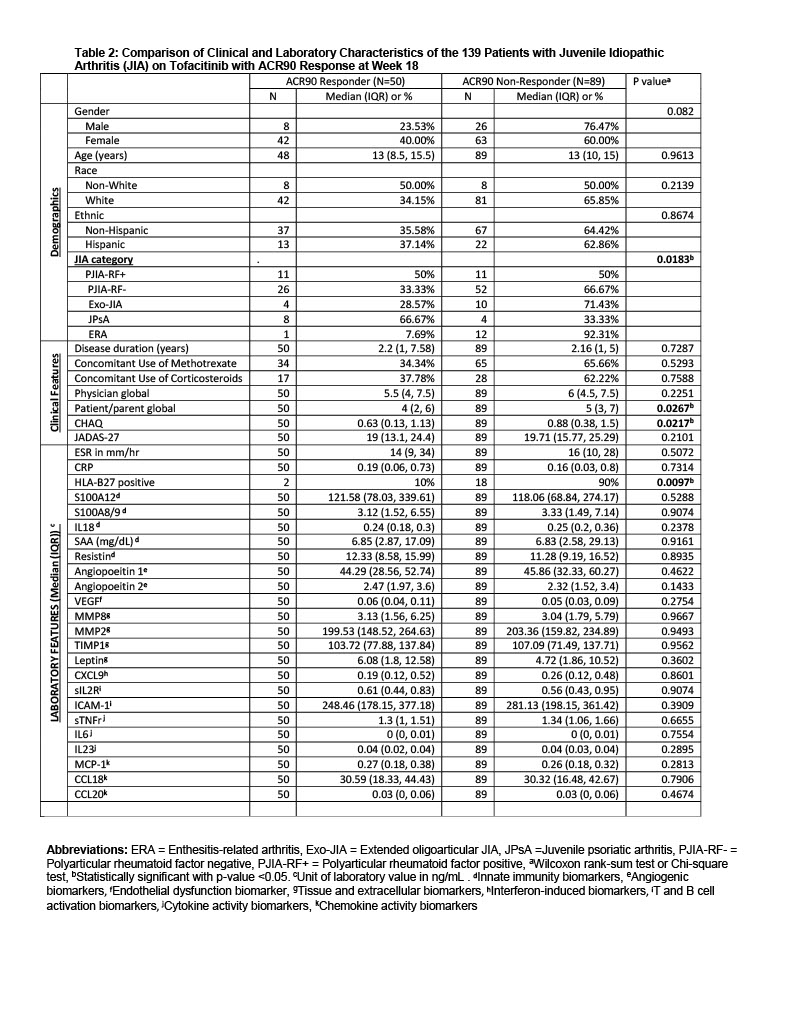
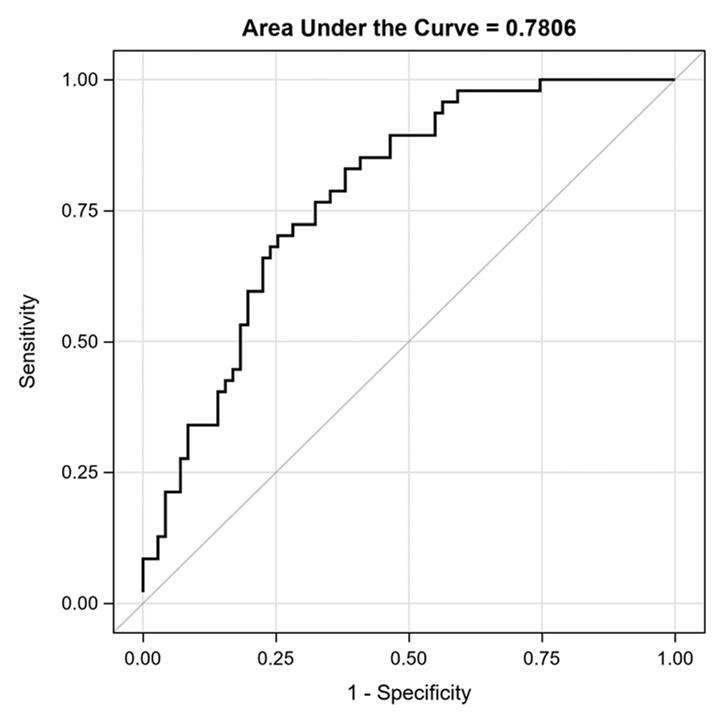 Figure 1: Receiver operating curve of logistic regression model with baseline IL-18, decrease in S100A12 and MCP-1 levels shows these candidate blood biomarkers as excellent predictors of ACR90 response in juvenile idiopathic arthritis patients on Tofacitinib
Figure 1: Receiver operating curve of logistic regression model with baseline IL-18, decrease in S100A12 and MCP-1 levels shows these candidate blood biomarkers as excellent predictors of ACR90 response in juvenile idiopathic arthritis patients on Tofacitinib
Disclosures: E. Ogbu, None; S. Thornton, None; A. Sproles, None; A. Grom, Sobi, Novartis; S. Dhakal, None; B. Huang, None; H. Brunner, Cincinnati Children's Hospital, Pfizer, GlaxoSmithKlein(GSK), AbbVie/Abbott, AstraZeneca, Medimmune, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, EMD Serono, Idorsia, Cerecor, Janssen, Roche, Merck/MSD, Novartis, R-Harm, Sanofi.
Background/Purpose: Tofacitinib has been shown to improve disease activity of several subtypes of Juvenile Idiopathic Arthritis (JIA). No known biomarkers to date predict JIA improvement on Tofacitinib. The purpose of this study was to test the usefulness of candidate blood-based biomarkers (CBB) previously associated with status and course of JIA or rheumatoid arthritis for prediction of JIA disease activity and improvement after 18 weeks of tofacitinib use.
Methods: Data and samples were from the phase 3 randomized clinical trial in JIA (NCT02592434). CBB studied were: S100A8/9, S100A12, IL18, SAA, resistin, VEGF, Angiopoietin-1, Angiopoietin-2, MMP8, MMP2, TIMP1, Leptin, CXCL9, soluble (s) IL2R, ICAM-1, sTNFR, IL6, IL23, MCP-1, CCL18, and CCL20. Samples of 166 patients aged 2-< 18 years with the following JIA subtypes were assayed: 1) polyarticular rheumatoid factor (RF) positive, 2) RF negative, 3) extended oligoarticular, 4) juvenile psoriatic arthritis,5) enthesitis-related arthritis , and 6) systemic (SJIA). All patients received tofacitinib from baseline to week 18 (BL-wk18). Outcomes of treatment response were: change in JIA disease activity score (JADAS27-CRP) and JIA-ACR90 level improvement (BL-wk18).
Results: Primary analysis excluded 7 SJIA patients, given the unique mechanism of autoinflammation in SJIA. Table 1 summarizes BL characteristics of the remaining 159 patients. BL median levels of select CBB differed between JIA subtypes (S100A12, IL18, resistin, VEGF, sTNFR, CCL20), as shown in Table 1. At wk18, samples of 139 patients were available for analysis, 50 (36%) achieved ACR90, 49/20/6 or 35%/14%/4% achieved ACR70/50/30. HLA-B27 positivity was associated with a significantly decreased odds of achieving a JIA-ACR90 level response (Table 2). Thus, the remaining analyses on CBB excluded N=21 HLA-B27 positive records. At BL, JADAS27-CRP was significantly correlated with IL6 (r = 0.24, p=0.004), IL18 (r = 0.18; p=0.03), SAA (r = 0.19; p=0.03), and MIG (0.18, p =0.03). Change of S100A12 significantly predicted the change in JADAS27-CRP (p = 0.0002) and in ACR90 [AUC=0.73, 95%CI of 0.64-0.82], after adjustments for BL JADAS27-CRP, JIA subtype, sex, age of diagnosis and disease duration. Similarly, BL-wk18 change in resistin and BL resistin significantly predicted the change in JADAS27-CRP [R-square=0.78] and in ACR_90 [AUC=0.72, 95%CI of 0.63-0.81]. Stepwise logistic selection identified that BL IL18, and decrease in S100A12 and MCP-1 were excellent predictors of JIA-ACR90 response (AUC: 0.78; 95% CI: 0.70-0.87; Figure 1). Secondary analysis including SJIA patients yielded similar findings. Background methotrexate and/or corticosteroids were not associated with CBB levels at BL or wk18.
Conclusion: While many previously proposed CBB did not predict response of JIA patients to tofacitinib, our exploratory analysis suggests that S100A12, MCP-1 may be predictive of or associated with JIA-ACR90 level improvement and improvement of JIA disease activity at wk18. Further, our analysis suggests that HLA B27 positivity may be associated with lower response to tofacitinib however numbers were small. Confirmation of these findings in an independent validation cohort is needed.


 Figure 1: Receiver operating curve of logistic regression model with baseline IL-18, decrease in S100A12 and MCP-1 levels shows these candidate blood biomarkers as excellent predictors of ACR90 response in juvenile idiopathic arthritis patients on Tofacitinib
Figure 1: Receiver operating curve of logistic regression model with baseline IL-18, decrease in S100A12 and MCP-1 levels shows these candidate blood biomarkers as excellent predictors of ACR90 response in juvenile idiopathic arthritis patients on TofacitinibDisclosures: E. Ogbu, None; S. Thornton, None; A. Sproles, None; A. Grom, Sobi, Novartis; S. Dhakal, None; B. Huang, None; H. Brunner, Cincinnati Children's Hospital, Pfizer, GlaxoSmithKlein(GSK), AbbVie/Abbott, AstraZeneca, Medimmune, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, EMD Serono, Idorsia, Cerecor, Janssen, Roche, Merck/MSD, Novartis, R-Harm, Sanofi.

