Back
Poster Session B
Session: (0807–0832) Miscellaneous Rheumatic and Inflammatory Diseases Poster II
0817: Predictive Severity Factors of COVID-19 in Patients with Rheumatic Immune Mediated Diseases
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- DM
David Martinez-Lopez, MD
Hospital Universitario Marques de Valdecilla
Santander (SPAIN), Spain
Abstract Poster Presenter(s)
David Martínez-López1, Ivan Ferraz Amaro2, Diana Prieto-Peña3, Fabricio Benavides Villanueva1, Cristina Corrales1, Lara Sánchez-Bilbao1, Alba Herrero-Morant4, Carmen Alvarez Reguera1, martín Trigueros-Vazquez1, Reinhard Wallmann5, Miguel Ángel González-Gay6 and Ricardo Blanco7, 1Hospital Universitario Marqués de Valdecilla, Santander, Spain, 2Division of Rheumatology. Hospital Universitario de Canarias. Spain., Santa Cruz de Tenerife, Spain, 3Research Group on Genetic Epidemiology and Atherosclerosis in Systemic Diseases and in Metabolic Bone Diseases of the Musculoskeletal System, IDIVAL; and Department of Rheumatology, Hospital Universitario Marqués de Valdecilla, Santander, Spain, 4Hospital Universitario Marqués de Valdecilla, Ontinyent, Spain, 5Servicio Cántabro de Salud, Santander, Spain, 6Department of Medicine and Psychiatry, Universidad de Cantabria; Rheumatology Division, Hospital Universitario Marqués de Valdecilla; Research group on genetic epidemiology and atherosclerosis in systemic diseases and in metabolic diseases of the musculoskeletal system, IDIVAL, Santander, Spain. Cardiovascular Pathophysiology and Genomics Research Unit, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa, 7Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain
Background/Purpose: COVID-19 has become a common disease in patients with rheumatic immune-mediated diseases (R-IMID). A risk stratification of the patients at COVID-19 onset is important to predict possible unfavorable results.
Our aim was to identify predictive severity factors in patients with COVID-19 with R-IMID.
Methods: Cross-sectional study in a single University Hospital. We included all consecutive patients with a R-IMID and COVID-19 up to November 6th, 2020. Confirmed infection was defined if the patient had a positive nasopharyngeal swab for SARS-CoV-2.
COVID-19 case severity was divided into mild, moderate, severe and critical according to the United States National Institute of Health (NIH) COVID-19 guidelines.
We performed a multivariable analysis and calculated de odds ratio of critical COVID in patients with R-IMID, adjusting by age, sex and comorbidities.
Results: We included 274 patients with R-IMID complicated with COVID-19. At COVID-19 onset, the main comorbidities, analytical values, underlying R-IMID and treatments received are shown in TABLE.
According to COVID-19 severity, patients were mild (n=209; 76.3%), moderate (n=35; 12.8%), severe (n=9; 3.3%) and critical (n=21; 7.7%).
The predictive variables at COVID-19 onset related statistically to critical COVID were older patients, hypertension, dyslipidemia, previous cardiovascular disease, cancer, chronic kidney disease, and chronic liver disease. The only underlying R-IMID and treatment was polymyalgia rheumatica and Rituximab, respectively. Regarding analytical values were higher values of C-reactive protein, LDH, platelets and lymphopenia (FIGURE).
Conclusion: We identified various factors associated with a worse prognosis of COVID-19 in patients with R-IMID. This can help to identify which patients can present a worse course of the disease at the moment of the diagnosis.
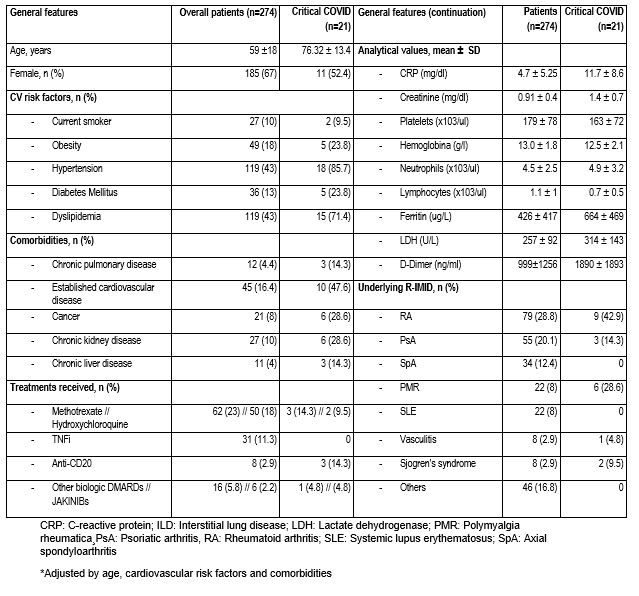 Table. General features patients with R-IMID and COVID-19
Table. General features patients with R-IMID and COVID-19
 FIGURE. Predictive factors for critical COVID-19 in R-IMID (Multivariable analysis)
FIGURE. Predictive factors for critical COVID-19 in R-IMID (Multivariable analysis)
Disclosures: D. Martínez-López, None; I. Ferraz Amaro, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Pfizer, Roche, Amgen, Celgene, Merck/MSD; D. Prieto-Peña, UCB, Roche, Pfizer, Amgen, Janssen, AbbVie/Abbott, Novartis, Eli Lilly; F. Benavides Villanueva, None; C. Corrales, None; L. Sánchez-Bilbao, Eli Lilly; A. Herrero-Morant, None; C. Alvarez Reguera, None; m. Trigueros-Vazquez, None; R. Wallmann, None; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.
Background/Purpose: COVID-19 has become a common disease in patients with rheumatic immune-mediated diseases (R-IMID). A risk stratification of the patients at COVID-19 onset is important to predict possible unfavorable results.
Our aim was to identify predictive severity factors in patients with COVID-19 with R-IMID.
Methods: Cross-sectional study in a single University Hospital. We included all consecutive patients with a R-IMID and COVID-19 up to November 6th, 2020. Confirmed infection was defined if the patient had a positive nasopharyngeal swab for SARS-CoV-2.
COVID-19 case severity was divided into mild, moderate, severe and critical according to the United States National Institute of Health (NIH) COVID-19 guidelines.
We performed a multivariable analysis and calculated de odds ratio of critical COVID in patients with R-IMID, adjusting by age, sex and comorbidities.
Results: We included 274 patients with R-IMID complicated with COVID-19. At COVID-19 onset, the main comorbidities, analytical values, underlying R-IMID and treatments received are shown in TABLE.
According to COVID-19 severity, patients were mild (n=209; 76.3%), moderate (n=35; 12.8%), severe (n=9; 3.3%) and critical (n=21; 7.7%).
The predictive variables at COVID-19 onset related statistically to critical COVID were older patients, hypertension, dyslipidemia, previous cardiovascular disease, cancer, chronic kidney disease, and chronic liver disease. The only underlying R-IMID and treatment was polymyalgia rheumatica and Rituximab, respectively. Regarding analytical values were higher values of C-reactive protein, LDH, platelets and lymphopenia (FIGURE).
Conclusion: We identified various factors associated with a worse prognosis of COVID-19 in patients with R-IMID. This can help to identify which patients can present a worse course of the disease at the moment of the diagnosis.
 Table. General features patients with R-IMID and COVID-19
Table. General features patients with R-IMID and COVID-19 FIGURE. Predictive factors for critical COVID-19 in R-IMID (Multivariable analysis)
FIGURE. Predictive factors for critical COVID-19 in R-IMID (Multivariable analysis) Disclosures: D. Martínez-López, None; I. Ferraz Amaro, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Pfizer, Roche, Amgen, Celgene, Merck/MSD; D. Prieto-Peña, UCB, Roche, Pfizer, Amgen, Janssen, AbbVie/Abbott, Novartis, Eli Lilly; F. Benavides Villanueva, None; C. Corrales, None; L. Sánchez-Bilbao, Eli Lilly; A. Herrero-Morant, None; C. Alvarez Reguera, None; m. Trigueros-Vazquez, None; R. Wallmann, None; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.

