Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0913–0938) RA – Treatment Poster II
0919: Predictors of Methotrexate Monotherapy Response in Patients with Active Rheumatoid Arthritis: Results from a Multicentre, Randomized Controlled Trial
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

Siddharth Jain, MD, MBBS, DM, MRCP
Postgraduate Institute of Medical Education and Research, Chandigarh, India
Chandigarh, Chandigarh, India
Abstract Poster Presenter(s)
Siddharth Jain1, Varun Dhir2, Amita Aggarwal3, Ranjan Gupta4, Bidya Laishangtham1, Aastha Khullar1, Shankar Naidu1, Veena Dhawan1, Shefali Sharma5, Aman Sharma6 and sanjay jain1, 1Postgraduate Institute of Medical Education and Research, Chandigarh, India, 2PGIMER, CHD, INDIA, Chandigarh, India, 3Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, 4All India Institute of Medical Sciences, New Delhi, India, 5PGIMER< Chandigarh, Chandigarh, India, 6PGIMER, Chandigarh, India, Chandigarh, India
Background/Purpose: Methotrexate (MTX) is the gold standard, first-line therapy for rheumatoid arthritis (RA). However, not all patients respond to MTX, and the predictors of its response or non-response have not yet been reliably identified. Identification of these predictors will facilitate personalized therapeutic choices, and improve patient outcomes. We thus sought to identify the demographic and clinico-laboratory predictors of response to MTX monotherapy in patients with active RA.
Methods: This study included patients with active RA (SJC≥2 and TJC≥4) aged 18-55 years, with disease duration < 5 years, who were not receiving DMARDs (except HCQ and low-dose prednisolone) and had been enrolled in the multicentre, parallel group RCT comparing two different MTX escalation strategies in RA (MEIRA). All these patients received MTX monotherapy which was started at 15 mg/week, escalated to 25 mg/week by 4-8 weeks, and continued till 16 weeks. MTX response was defined as EULAR good or moderate response (based on DAS28-3v) at 16 weeks. Stepwise, multivariable logistic regression was done using key demographic (age, gender, BMI, comorbidities), clinical (disease duration, DAS28, HAQ), and laboratory parameters (RF, anti-CCP, ESR, CRP, RBC MTX-polyglutamates 1-4, IL-6, MMP-3) as independent variables to identify predictors of MTX response. A two-tailed p-value < 0.05 was used for defining statistical significance. (Trial Reg: CTRI/2018/12/016549)
Results: Out of a total of 178 included patients [84% females, mean age 40 (9) years, mean DAS28-CRP=5.4 (1.1)], 113 (63.5%) were classified as MTX responders at 16 weeks. Age (OR=0.95, p=0.01), BMI (OR=1.12, p=0.006), and RF (OR=0.34, p=0.045) were found to be independent predictors of MTX response on multivariable analysis (Table 1). On sensitivity analysis with DAS28-ESR-based EULAR response, age (OR=0.94, p=0.003) and RF (OR=0.42, p=0.059) were replicated as independent predictors of MTX response, in addition to pre-treatment swollen joint count (OR=0.94, p=0.05).
Conclusion: Younger age, RF negativity, higher BMI, and lower pre-treatment swollen joint count are potential predictors of response to MTX monotherapy in RA.
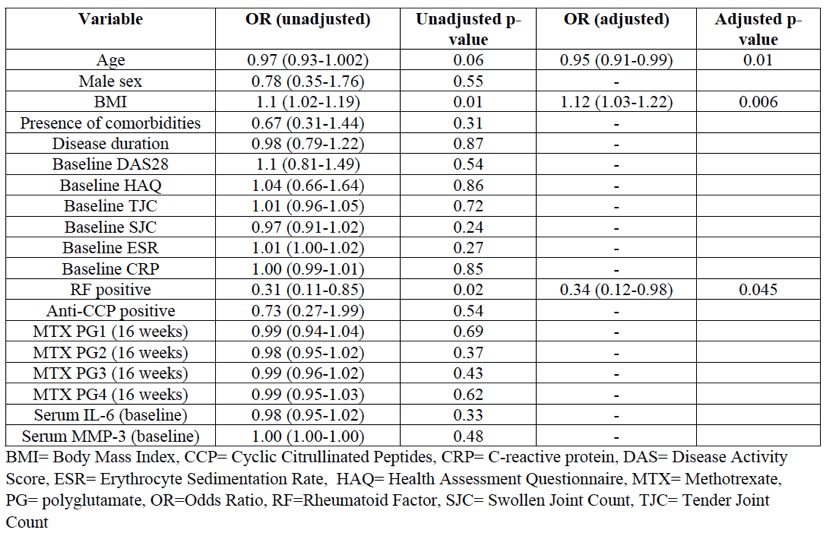 Table 1: Results of multivariable logistic regression analysis for prediction of response (as defined by DAS28-CRP-based EULAR good or moderate response) to methotrexate monotherapy in RA.
Table 1: Results of multivariable logistic regression analysis for prediction of response (as defined by DAS28-CRP-based EULAR good or moderate response) to methotrexate monotherapy in RA.
Disclosures: S. Jain, None; V. Dhir, None; A. Aggarwal, None; R. Gupta, None; B. Laishangtham, None; A. Khullar, None; S. Naidu, None; V. Dhawan, None; S. Sharma, None; A. Sharma, None; s. jain, None.
Background/Purpose: Methotrexate (MTX) is the gold standard, first-line therapy for rheumatoid arthritis (RA). However, not all patients respond to MTX, and the predictors of its response or non-response have not yet been reliably identified. Identification of these predictors will facilitate personalized therapeutic choices, and improve patient outcomes. We thus sought to identify the demographic and clinico-laboratory predictors of response to MTX monotherapy in patients with active RA.
Methods: This study included patients with active RA (SJC≥2 and TJC≥4) aged 18-55 years, with disease duration < 5 years, who were not receiving DMARDs (except HCQ and low-dose prednisolone) and had been enrolled in the multicentre, parallel group RCT comparing two different MTX escalation strategies in RA (MEIRA). All these patients received MTX monotherapy which was started at 15 mg/week, escalated to 25 mg/week by 4-8 weeks, and continued till 16 weeks. MTX response was defined as EULAR good or moderate response (based on DAS28-3v) at 16 weeks. Stepwise, multivariable logistic regression was done using key demographic (age, gender, BMI, comorbidities), clinical (disease duration, DAS28, HAQ), and laboratory parameters (RF, anti-CCP, ESR, CRP, RBC MTX-polyglutamates 1-4, IL-6, MMP-3) as independent variables to identify predictors of MTX response. A two-tailed p-value < 0.05 was used for defining statistical significance. (Trial Reg: CTRI/2018/12/016549)
Results: Out of a total of 178 included patients [84% females, mean age 40 (9) years, mean DAS28-CRP=5.4 (1.1)], 113 (63.5%) were classified as MTX responders at 16 weeks. Age (OR=0.95, p=0.01), BMI (OR=1.12, p=0.006), and RF (OR=0.34, p=0.045) were found to be independent predictors of MTX response on multivariable analysis (Table 1). On sensitivity analysis with DAS28-ESR-based EULAR response, age (OR=0.94, p=0.003) and RF (OR=0.42, p=0.059) were replicated as independent predictors of MTX response, in addition to pre-treatment swollen joint count (OR=0.94, p=0.05).
Conclusion: Younger age, RF negativity, higher BMI, and lower pre-treatment swollen joint count are potential predictors of response to MTX monotherapy in RA.
 Table 1: Results of multivariable logistic regression analysis for prediction of response (as defined by DAS28-CRP-based EULAR good or moderate response) to methotrexate monotherapy in RA.
Table 1: Results of multivariable logistic regression analysis for prediction of response (as defined by DAS28-CRP-based EULAR good or moderate response) to methotrexate monotherapy in RA.Disclosures: S. Jain, None; V. Dhir, None; A. Aggarwal, None; R. Gupta, None; B. Laishangtham, None; A. Khullar, None; S. Naidu, None; V. Dhawan, None; S. Sharma, None; A. Sharma, None; s. jain, None.

