Back
Abstract Session
Session: Abstracts: RA – Diagnosis, Manifestations, and Outcomes III: RA ILD (2248–2253)
2249: Impact of Interstitial Lung Disease on COVID-19 Severity Among Patients with Rheumatoid Arthritis: A Multicenter Comparative Study
Monday, November 14, 2022
4:45 PM – 4:55 PM Eastern Time
Location: Room 103
- GF
Gabriel Figueroa-Parra, MD
Mayo Clinic
Rochester, MN, United States
Presenting Author(s)
Gabriel Figueroa Parra1, Emily Gilbert2, Maria Valenzuela Almada1, Sebastian Vallejo1, Matthew Neville3, Naomi Patel4, Claire Cook5, Xiaoqing Fu5, Ramla Hagi5, Gregory McDermott6, Michael Dilorio6, Lucy Masto6, Kathleen Vanni6, Emily Kowalski6, Grace Qian6, Zachary Wallace5, Ali Duarte-Garcia1 and Jeffrey Sparks7, 1Mayo Clinic, Rochester, MN, 2Mayo Clinic, Jacksonville, FL, 3Mayo Clinic, Phoenix, AZ, 4Massachusetts General Hospital, Sale Creek, TN, 5Massachusetts General Hospital, Boston, MA, 6Brigham and Women's Hospital, Boston, MA, 7Brigham and Women's Hospital and Harvard Medical School, Boston, MA
Background/Purpose: Rheumatoid arthritis (RA) has been associated with severe COVID-19, but few studies have investigated outcomes in RA phenotypes such as interstitial lung disease (RA-ILD), seropositivity, and bone erosions. We aimed to assess COVID-19 outcomes in patients with RA overall, and those with and without RA-ILD, compared to general population comparators.
Methods: A multicenter, retrospective comparative cohort study was conducted at two healthcare systems with hospitals and outpatient centers in 6 states across the US. Consecutive patients with RA meeting 2010 ACR/EULAR criteria and a positive COVID-19 test (index date) from March 1, 2020, through June 6, 2021, were matched 1:5 on age, sex, race, and calendar date to general population comparators without RA. We examined subgroups of RA by phenotypic features: RA-ILD per Bongartz criteria (Bongartz, T. Arthritis Rheum. 2010), serostatus (either rheumatoid factor or cyclic citrullinated peptide antibody), and bone erosions. Severe COVID-19 was a composite of hospitalization or death within 14 days of COVID-19 diagnosis. We used multivariable Cox regression to investigate the association of RA and RA phenotypes with COVID-19 outcomes compared to matched comparators.
Results: We identified 582 patients with RA and 2,875 matched comparators, all with COVID-19. The mean age was 62 years, 72% were female, and 79% were White. Among patients with RA, median RA duration was 8 years, 9% had RA-ILD, 68% were seropositive, 27% had bone erosions. 28% were using glucocorticoids, and 79% were on DMARDs at the time of COVID-19 diagnosis. During a follow-up of 41,411 person-days, there were 126 (22%) severe outcomes observed in the RA patients and 363 (13%) in the comparators (follow-up 226,550 person-days). The corresponding rate was higher among RA patients than comparators (3.0 per 1,000 days [95% CI 2.5-3.6] vs. 1.6 per 1,000 days [95% CI 1.4-1.8], respectively). Overall, RA patients had a 75% higher risk of severe COVID-19 than comparators after adjustment (HR 1.75, 95% CI 1.45-2.10). Among those with RA-ILD, the severe COVID-19 rate was significantly higher than their comparators (10.9 [95% CI 6.7-15.2] vs. 2.5 per 1,000 days [1.8-3.2], respectively). RA-ILD was associated with 2.5-fold higher risk for severe COVID-19 than comparators (multivariable HR 2.50 [95% CI 1.66-3.77], Table). There was a significant interaction between RA/comparator status and presence/absence of ILD for risk of severe COVID-19 (p< 0.046, Figure). The elevated risk for severe COVID-19 was similarly higher for seropositive and erosive RA compared to the general population but these were not effect modifiers of the association of RA with severe outcomes.
Conclusion: RA patients have a higher risk of severe COVID-19, especially those with ILD. Our findings suggest that ILD or its treatment may be a significant contributor to severe COVID-19 outcomes in RA.
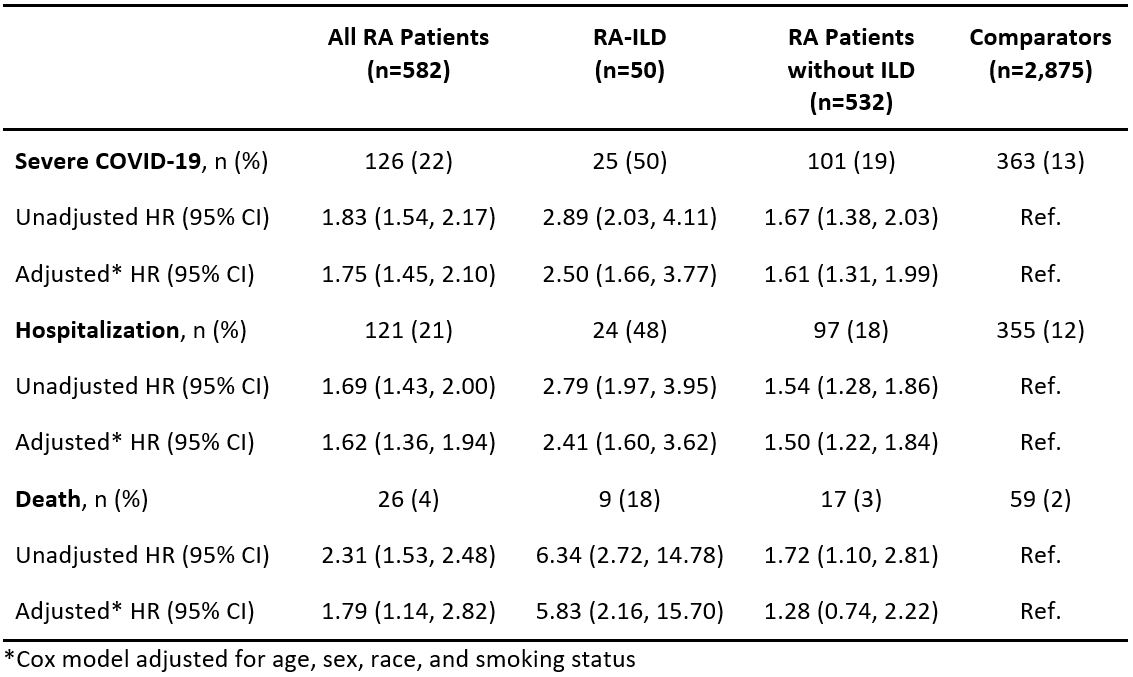 Table. Frequencies, proportions, and hazard ratios for COVID-19 outcomes, comparing all RA patients, and subgroups with or without RA-ILD, to matched comparators.
Table. Frequencies, proportions, and hazard ratios for COVID-19 outcomes, comparing all RA patients, and subgroups with or without RA-ILD, to matched comparators.
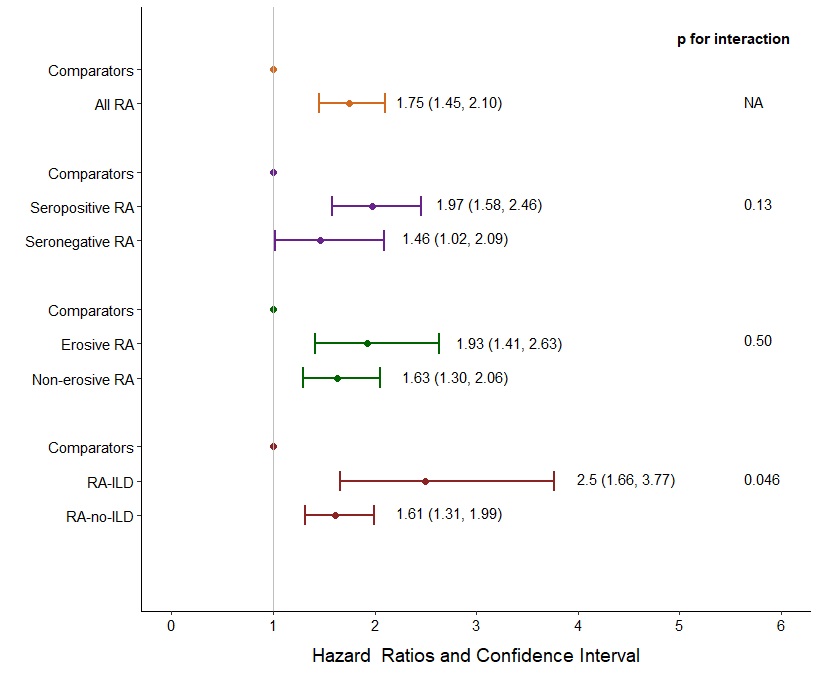 Figure. Multivariable hazard ratios for severe COVID-19, comparing all RA and subgroups by serostatus, bone erosions, and RA-ILD to matched comparators without RA. Cox model adjusted for age, sex, race and smoking status.
Figure. Multivariable hazard ratios for severe COVID-19, comparing all RA and subgroups by serostatus, bone erosions, and RA-ILD to matched comparators without RA. Cox model adjusted for age, sex, race and smoking status.
Disclosures: G. Figueroa Parra, None; E. Gilbert, None; M. Valenzuela Almada, None; S. Vallejo, None; M. Neville, None; N. Patel, FVC Health; C. Cook, None; X. Fu, None; R. Hagi, None; G. McDermott, None; M. Dilorio, None; L. Masto, None; K. Vanni, None; E. Kowalski, None; G. Qian, None; Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Shionogi, Horizon; A. Duarte-Garcia, None; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer.
Background/Purpose: Rheumatoid arthritis (RA) has been associated with severe COVID-19, but few studies have investigated outcomes in RA phenotypes such as interstitial lung disease (RA-ILD), seropositivity, and bone erosions. We aimed to assess COVID-19 outcomes in patients with RA overall, and those with and without RA-ILD, compared to general population comparators.
Methods: A multicenter, retrospective comparative cohort study was conducted at two healthcare systems with hospitals and outpatient centers in 6 states across the US. Consecutive patients with RA meeting 2010 ACR/EULAR criteria and a positive COVID-19 test (index date) from March 1, 2020, through June 6, 2021, were matched 1:5 on age, sex, race, and calendar date to general population comparators without RA. We examined subgroups of RA by phenotypic features: RA-ILD per Bongartz criteria (Bongartz, T. Arthritis Rheum. 2010), serostatus (either rheumatoid factor or cyclic citrullinated peptide antibody), and bone erosions. Severe COVID-19 was a composite of hospitalization or death within 14 days of COVID-19 diagnosis. We used multivariable Cox regression to investigate the association of RA and RA phenotypes with COVID-19 outcomes compared to matched comparators.
Results: We identified 582 patients with RA and 2,875 matched comparators, all with COVID-19. The mean age was 62 years, 72% were female, and 79% were White. Among patients with RA, median RA duration was 8 years, 9% had RA-ILD, 68% were seropositive, 27% had bone erosions. 28% were using glucocorticoids, and 79% were on DMARDs at the time of COVID-19 diagnosis. During a follow-up of 41,411 person-days, there were 126 (22%) severe outcomes observed in the RA patients and 363 (13%) in the comparators (follow-up 226,550 person-days). The corresponding rate was higher among RA patients than comparators (3.0 per 1,000 days [95% CI 2.5-3.6] vs. 1.6 per 1,000 days [95% CI 1.4-1.8], respectively). Overall, RA patients had a 75% higher risk of severe COVID-19 than comparators after adjustment (HR 1.75, 95% CI 1.45-2.10). Among those with RA-ILD, the severe COVID-19 rate was significantly higher than their comparators (10.9 [95% CI 6.7-15.2] vs. 2.5 per 1,000 days [1.8-3.2], respectively). RA-ILD was associated with 2.5-fold higher risk for severe COVID-19 than comparators (multivariable HR 2.50 [95% CI 1.66-3.77], Table). There was a significant interaction between RA/comparator status and presence/absence of ILD for risk of severe COVID-19 (p< 0.046, Figure). The elevated risk for severe COVID-19 was similarly higher for seropositive and erosive RA compared to the general population but these were not effect modifiers of the association of RA with severe outcomes.
Conclusion: RA patients have a higher risk of severe COVID-19, especially those with ILD. Our findings suggest that ILD or its treatment may be a significant contributor to severe COVID-19 outcomes in RA.
 Table. Frequencies, proportions, and hazard ratios for COVID-19 outcomes, comparing all RA patients, and subgroups with or without RA-ILD, to matched comparators.
Table. Frequencies, proportions, and hazard ratios for COVID-19 outcomes, comparing all RA patients, and subgroups with or without RA-ILD, to matched comparators. Figure. Multivariable hazard ratios for severe COVID-19, comparing all RA and subgroups by serostatus, bone erosions, and RA-ILD to matched comparators without RA. Cox model adjusted for age, sex, race and smoking status.
Figure. Multivariable hazard ratios for severe COVID-19, comparing all RA and subgroups by serostatus, bone erosions, and RA-ILD to matched comparators without RA. Cox model adjusted for age, sex, race and smoking status.Disclosures: G. Figueroa Parra, None; E. Gilbert, None; M. Valenzuela Almada, None; S. Vallejo, None; M. Neville, None; N. Patel, FVC Health; C. Cook, None; X. Fu, None; R. Hagi, None; G. McDermott, None; M. Dilorio, None; L. Masto, None; K. Vanni, None; E. Kowalski, None; G. Qian, None; Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Shionogi, Horizon; A. Duarte-Garcia, None; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer.

