Back
Abstract Session
Session: Abstracts: Muscle Biology, Myositis and Myopathies (2237–2242)
2238: Performance of the 2016 ACR/EULAR Myositis Response Criteria in Adult Dermatomyositis and Polymyositis Therapeutic Trials and Consensus Profiles
Monday, November 14, 2022
4:45 PM – 4:55 PM Eastern Time
Location: Room 201

Didem Saygin, MD
University of Pittsburgh
Pittsburgh, PA, United States
Presenting Author(s)
Didem Saygin1, Hanna Kim2, Christian Douglas3, Brian Erman3, Jesse Wilkerson3, john mcgrath3, Chester Oddis4, Ingrid Lundberg5, Anthony Amato6, Ignacio Garcia-De La Torre7, Hector Chinoy8, David Fiorentino9, Lorinda Chung9, Yeong-Wook Song10, Katalin Danko11, Frederick Miller12, Nicola Ruperto13, Jiří Vencovský14, Rohit Aggarwal15 and Lisa G Rider12, 1University of Chicago, Chicago, IL, 2Division of Rheumatology, Department of Medicine, George Washington University School of Medicine and Health Sciences; Juvenile Myositis Therapeutic and Translation Studies Unit, PTRB, NIAMS, NIH, Bethesda, MD, 3Social and Scientific Systems, Inc., Durham, NC, 4University of Pittsburgh, Pittsburgh, PA, 5Karolinska Universitetssjukhuset, Karolinska Institutet, Stockholm, Sweden, 6Harvard University, Boston, MA, 7Centro de Est. de Inv. Bas. y Clinica, S.C., Guadalajara, Mexico, 8The University of Manchester, Sale, United Kingdom, 9Stanford University, Stanford, CA, 10Seoul National University, Seoul, Republic of Korea, 11*Deceased, Debrecen, Hungary, 12Environmental Autoimmunity Group, Clinical Research Branch, National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health, Bethesda, MD, 13IRCCS Istituto Giannina Gaslini; PRINTO, Clinica Pediatrica e Reumatologia, Genova, Italy, 14Institute of Rheumatology and Department of Rheumatology, First Faculty of Medicine, Charles University, Prague, Czech Republic, 15Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA
Background/Purpose: The ACR/EULAR myositis response criteria (MRC) were developed as a composite continuous measure (Total Improvement Score (TIS)) using absolute percent changes (abs%) in 6 differentially weighted core set measures (CSM), providing improvement categories (minimal, moderate, major) based on pre-specified thresholds. It is currently unclear whether patients can achieve response per MRC without improvement in strength or with worsening in any CSMs, a concern to regulatory agencies. Representation of patient reported outcome measures (PRO) also remains to be examined. In this study, we aimed to assess the contribution of each CSM to TIS, frequency of worsening in CSMs, strength vs. extramuscular global activity (ExtraMusc) improvement and reflection of PRO in TIS.
Methods: Data from adult DM/PM patients enrolled in the Rituximab in Myositis (n=147), Etanercept in DM (n=14), and Abatacept Treatment in PM/DM (n=19) trials, and consensus profiles from natural history and open-label treatment studies (n=232) were included. The number of improving/worsening CSMs by MRC category was examined. Frequency of improvement with and without muscle-related CSMs (manual muscle testing (MMT), Health Assessment Questionnaire (HAQ), and/or muscle enzyme), and PROM (HAQ and Patient Global Disease Activity (PTGA)) was calculated. Wilcoxon test with Bonferroni adjusted p value was performed for comparison among MRC categories. Regression analysis was performed to test the contribution of each CSM. Sign test was used to compare the observed vs expected contribution of each CSM. Physician assessed change categories were compared to MRC categories with weighted Cohen's κ test.
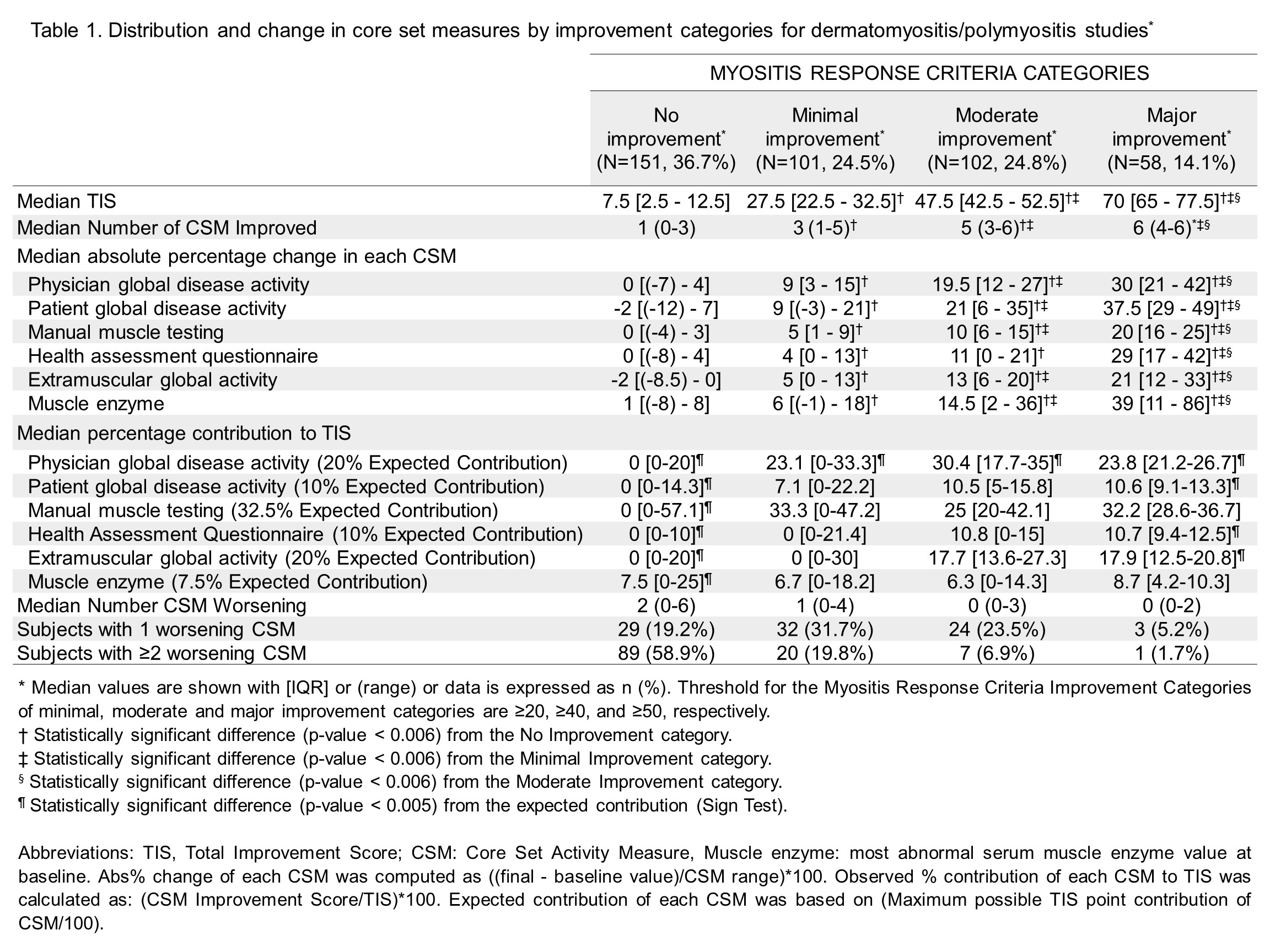
Results: Of 412 adults with DM/PM, there were 36.7%, 24.5%, 24.8%, and 14.1% with no, minimal, moderate, and major improvement by MRC, respectively (Table 1). As the level of improvement increased from minimal to major, the number of improving CSMs and abs% change in all CSMs significantly increased. In minimal-moderate improvement, only physician global contributed significantly more than expected, and other CSM contributed as expected. With the exception of muscle enzyme level, changes in each CSM contributed significantly to the TIS. Patients with no-minimal improvement had a median of 1-2 worsening CSMs, whereas patients with moderate-major improvement had a median of zero worsening CSM. Of patients with at least minimal improvement, 95% had improvement in muscle related measures and 84% had improvement in PRO (Figure 1). Conversely, only 4% had improvement in ExtraMusc without improvement in muscle related measures. In general, physician assessed change showed significant agreement with the MRC categories with Cohen's κ of 0.53 for Rituximab trial and 0.79 for consensus profiles.
Conclusion: This study demonstrated that each CSM significantly contributes to the TIS, with the exception of muscle enzyme. The majority of DM/PM patients who improve by the MRC show improvement in muscle disease, while it is uncommon to meet MRC improvement without improvement in muscle disease. The MRC categories infrequently show worsening in any CSMs and often reflect improvement in PRO. The ACR-EULAR MRC are robust and perform consistently across multiple studies.

Disclosures: D. Saygin, None; H. Kim, Eli Lilly and Company; C. Douglas, None; B. Erman, None; J. Wilkerson, None; j. mcgrath, None; C. Oddis, None; I. Lundberg, Argenx, AstraZeneca, Bristol Myers Squibb, Novartis, Corbus, EMD Serono, Roche, Pfizer, Orphazyme, Octapharma, Kezar, Janssen; A. Amato, Abcuro, ArgenX, Ra pharmaceuticals, Horizon Therapeutics, OnoPharma, Alexion, EMD Serono, Takeda, Johnson & Johnson (COVID19 vaccination program); I. Garcia-De La Torre, None; H. Chinoy, Eli Lilly, UCB; D. Fiorentino, None; L. Chung, Kyverna, Mitsubishi Tanabe, Eicos, Boehringer-Ingelheim, Jasper, Genentech; Y. Song, None; K. Danko, None; F. Miller, None; N. Ruperto, 2 Bridge, Amgen, AstraZeneca, Aurinia, Bayer, Brystol Myers and Squibb, Celgene, inMed, Cambridge Healthcare Research, Domain Therapeutic,, EMD Serono, Glaxo Smith Kline, Idorsia, Janssen, Eli Lilly, Novartis, Pfizer, Sobi, UCB; J. Vencovský, Abbvie, Biogen, Boehringer, Eli Lilly, Gilead, Kezar, Merck, Novartis, Octapharma, Pfizer, Takeda, UCB, Werfen, Argenx; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech; L. Rider, Hope Pharmaceuticals, Pfizer, Bristol-Myers Squibb(BMS).
Background/Purpose: The ACR/EULAR myositis response criteria (MRC) were developed as a composite continuous measure (Total Improvement Score (TIS)) using absolute percent changes (abs%) in 6 differentially weighted core set measures (CSM), providing improvement categories (minimal, moderate, major) based on pre-specified thresholds. It is currently unclear whether patients can achieve response per MRC without improvement in strength or with worsening in any CSMs, a concern to regulatory agencies. Representation of patient reported outcome measures (PRO) also remains to be examined. In this study, we aimed to assess the contribution of each CSM to TIS, frequency of worsening in CSMs, strength vs. extramuscular global activity (ExtraMusc) improvement and reflection of PRO in TIS.
Methods: Data from adult DM/PM patients enrolled in the Rituximab in Myositis (n=147), Etanercept in DM (n=14), and Abatacept Treatment in PM/DM (n=19) trials, and consensus profiles from natural history and open-label treatment studies (n=232) were included. The number of improving/worsening CSMs by MRC category was examined. Frequency of improvement with and without muscle-related CSMs (manual muscle testing (MMT), Health Assessment Questionnaire (HAQ), and/or muscle enzyme), and PROM (HAQ and Patient Global Disease Activity (PTGA)) was calculated. Wilcoxon test with Bonferroni adjusted p value was performed for comparison among MRC categories. Regression analysis was performed to test the contribution of each CSM. Sign test was used to compare the observed vs expected contribution of each CSM. Physician assessed change categories were compared to MRC categories with weighted Cohen's κ test.
Results: Of 412 adults with DM/PM, there were 36.7%, 24.5%, 24.8%, and 14.1% with no, minimal, moderate, and major improvement by MRC, respectively (Table 1). As the level of improvement increased from minimal to major, the number of improving CSMs and abs% change in all CSMs significantly increased. In minimal-moderate improvement, only physician global contributed significantly more than expected, and other CSM contributed as expected. With the exception of muscle enzyme level, changes in each CSM contributed significantly to the TIS. Patients with no-minimal improvement had a median of 1-2 worsening CSMs, whereas patients with moderate-major improvement had a median of zero worsening CSM. Of patients with at least minimal improvement, 95% had improvement in muscle related measures and 84% had improvement in PRO (Figure 1). Conversely, only 4% had improvement in ExtraMusc without improvement in muscle related measures. In general, physician assessed change showed significant agreement with the MRC categories with Cohen's κ of 0.53 for Rituximab trial and 0.79 for consensus profiles.
Conclusion: This study demonstrated that each CSM significantly contributes to the TIS, with the exception of muscle enzyme. The majority of DM/PM patients who improve by the MRC show improvement in muscle disease, while it is uncommon to meet MRC improvement without improvement in muscle disease. The MRC categories infrequently show worsening in any CSMs and often reflect improvement in PRO. The ACR-EULAR MRC are robust and perform consistently across multiple studies.


Disclosures: D. Saygin, None; H. Kim, Eli Lilly and Company; C. Douglas, None; B. Erman, None; J. Wilkerson, None; j. mcgrath, None; C. Oddis, None; I. Lundberg, Argenx, AstraZeneca, Bristol Myers Squibb, Novartis, Corbus, EMD Serono, Roche, Pfizer, Orphazyme, Octapharma, Kezar, Janssen; A. Amato, Abcuro, ArgenX, Ra pharmaceuticals, Horizon Therapeutics, OnoPharma, Alexion, EMD Serono, Takeda, Johnson & Johnson (COVID19 vaccination program); I. Garcia-De La Torre, None; H. Chinoy, Eli Lilly, UCB; D. Fiorentino, None; L. Chung, Kyverna, Mitsubishi Tanabe, Eicos, Boehringer-Ingelheim, Jasper, Genentech; Y. Song, None; K. Danko, None; F. Miller, None; N. Ruperto, 2 Bridge, Amgen, AstraZeneca, Aurinia, Bayer, Brystol Myers and Squibb, Celgene, inMed, Cambridge Healthcare Research, Domain Therapeutic,, EMD Serono, Glaxo Smith Kline, Idorsia, Janssen, Eli Lilly, Novartis, Pfizer, Sobi, UCB; J. Vencovský, Abbvie, Biogen, Boehringer, Eli Lilly, Gilead, Kezar, Merck, Novartis, Octapharma, Pfizer, Takeda, UCB, Werfen, Argenx; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech; L. Rider, Hope Pharmaceuticals, Pfizer, Bristol-Myers Squibb(BMS).

