Back
Poster Session B
Epidemiology, health policy and outcomes
Session: (0724–0751) Health Services Research Poster II
0741: Prescribing Patterns of SGLT2 Inhibitors for Patients with Autoimmune Rheumatic Disease
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- EO
Emily G. Oakes, BA
Brigham and Women's Hospital
Boxford, MA, United States
Abstract Poster Presenter(s)
Emily G Oakes1, Jack Ellrodt1, May Choi2, Hongshu Guan1 and Karen Costenbader1, 1Brigham and Women's Hospital, Boston, MA, 2Brigham and Women's Hospital | University of Calgary, Calgary, AB, Canada
Background/Purpose: Sodium-glucose co-transporter 2 inhibitors (SGLT2i, dapagliflozin, canagliflozin, and empagliflozin) are a class of oral hypoglycemic medication for management of Type II diabetes mellitus (T2D), also demonstrated to lead to weight loss, decrease atherosclerotic cardiovascular risk, improve heart failure, reduce proteinuria, and prevent progression to end-stage renal disease (ESRD) in patients with chronic kidney disease. SGLT2i could have multiple benefits for patients with autoimmune rheumatic diseases (ARD), however these patients have not been included in clinical trials. Thus, we examined recent SGLT2i prescribing patterns for patients with ARD compared to patients without ARDs.
Methods: Using a centralized data registry from a large multihospital healthcare system we identified subjects with a ≥2 ICD-10 diagnostic codes for at least one of eight autoimmune rheumatic diseases prescribed dapagliflozin, canagliflozin, or empagliflozin between 1/1/2016 and 12/10/2021. Each ARD patient was matched by age, sex, and race to a control without ARD who was prescribed the same SGLT2i. Demographic and clinical data were collected on all subjects via data registry requests and electronic medical record review. Data comparison was conducted using Fisher exact tests and Chi-square analysis.
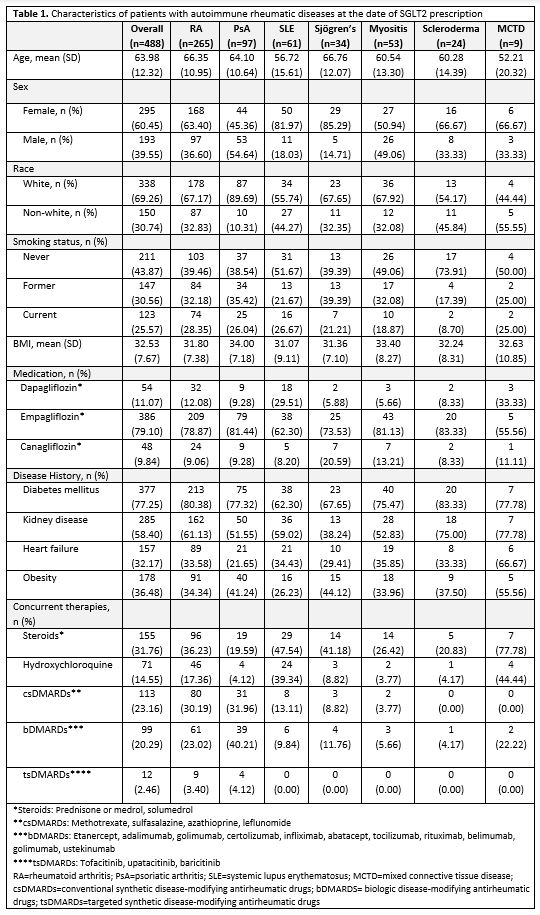
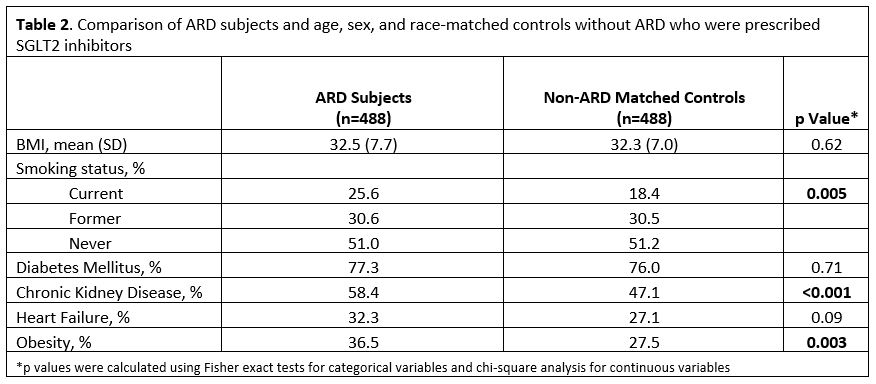
Results: We identified 488 total patients with ARD, including 265 rheumatoid arthritis, 97 psoriatic arthritis, 61 systemic lupus erythematosus, 34 Sjögren's, 53 inflammatory myositis, 24 scleroderma, and 9 mixed connective tissue disease. 386 were prescribed empagliflozin, 54 dapagliflozin, and 48 canagliflozin. The majority (77.25%) of this ARD population had T2D. A large proportion had kidney disease (58.40%), obesity (36.48%), and heart failure (32.17%) at the time of SGLT2i prescription (Table 1.). When compared to matched controls without ARD, ARD patients had more obesity (p=0.003), CKD (p=< 0.001), and heart failure (p=0.09) (Table 2.).
Conclusion: To our knowledge, this is the first study to examine prescribing patterns of SGLT2i in individuals with systemic ARD, a population historically excluded from studies of this nature. These findings suggest that SGLT2i may be prescribed in cases of more serious illness in patients with ARD compared to patients without ARD. More research is needed to identify differences in outcomes between these two populations to justify these differences in prescribing patterns.
Disclosures: E. Oakes, None; J. Ellrodt, None; M. Choi, AstraZeneca, MitogenDx, mallinckrodt, Janssen, AbbVie/Abbott, Alimentiv, Amgen, AVIR Pharma Inc, BioJAMP, Bristol-Myers Squibb(BMS), Celltrion, Ferring, Fresenius Kabi, McKesson, Mylan, Takeda, Pendopharm, Pfizer, Roche; H. Guan, None; K. Costenbader, Eli Lilly, Janssen, Amgen, AstraZeneca Pharmaceuticals LP, GlaxoSmithKline(GSK), Gilead, Exagen, Neutrolis, Cel-Sci, Alkermes.
Background/Purpose: Sodium-glucose co-transporter 2 inhibitors (SGLT2i, dapagliflozin, canagliflozin, and empagliflozin) are a class of oral hypoglycemic medication for management of Type II diabetes mellitus (T2D), also demonstrated to lead to weight loss, decrease atherosclerotic cardiovascular risk, improve heart failure, reduce proteinuria, and prevent progression to end-stage renal disease (ESRD) in patients with chronic kidney disease. SGLT2i could have multiple benefits for patients with autoimmune rheumatic diseases (ARD), however these patients have not been included in clinical trials. Thus, we examined recent SGLT2i prescribing patterns for patients with ARD compared to patients without ARDs.
Methods: Using a centralized data registry from a large multihospital healthcare system we identified subjects with a ≥2 ICD-10 diagnostic codes for at least one of eight autoimmune rheumatic diseases prescribed dapagliflozin, canagliflozin, or empagliflozin between 1/1/2016 and 12/10/2021. Each ARD patient was matched by age, sex, and race to a control without ARD who was prescribed the same SGLT2i. Demographic and clinical data were collected on all subjects via data registry requests and electronic medical record review. Data comparison was conducted using Fisher exact tests and Chi-square analysis.
Results: We identified 488 total patients with ARD, including 265 rheumatoid arthritis, 97 psoriatic arthritis, 61 systemic lupus erythematosus, 34 Sjögren's, 53 inflammatory myositis, 24 scleroderma, and 9 mixed connective tissue disease. 386 were prescribed empagliflozin, 54 dapagliflozin, and 48 canagliflozin. The majority (77.25%) of this ARD population had T2D. A large proportion had kidney disease (58.40%), obesity (36.48%), and heart failure (32.17%) at the time of SGLT2i prescription (Table 1.). When compared to matched controls without ARD, ARD patients had more obesity (p=0.003), CKD (p=< 0.001), and heart failure (p=0.09) (Table 2.).
Conclusion: To our knowledge, this is the first study to examine prescribing patterns of SGLT2i in individuals with systemic ARD, a population historically excluded from studies of this nature. These findings suggest that SGLT2i may be prescribed in cases of more serious illness in patients with ARD compared to patients without ARD. More research is needed to identify differences in outcomes between these two populations to justify these differences in prescribing patterns.


Disclosures: E. Oakes, None; J. Ellrodt, None; M. Choi, AstraZeneca, MitogenDx, mallinckrodt, Janssen, AbbVie/Abbott, Alimentiv, Amgen, AVIR Pharma Inc, BioJAMP, Bristol-Myers Squibb(BMS), Celltrion, Ferring, Fresenius Kabi, McKesson, Mylan, Takeda, Pendopharm, Pfizer, Roche; H. Guan, None; K. Costenbader, Eli Lilly, Janssen, Amgen, AstraZeneca Pharmaceuticals LP, GlaxoSmithKline(GSK), Gilead, Exagen, Neutrolis, Cel-Sci, Alkermes.

