Back
Poster Session B
Epidemiology, health policy and outcomes
Session: (0695–0723) Epidemiology and Public Health Poster I
0714: Real-World Treatment Effectiveness of Disease-Modifying Anti-Rheumatic Drugs by Serostatus Among Patients with Rheumatoid Arthritis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- YJ
Yinzhu Jin, MS
Brigham and Women's Hospital
Boston, MA, United States
Abstract Poster Presenter(s)
Yinzhu Jin, Jun Liu, Rishi Desai and Seoyoung Kim, Brigham and Women's Hospital, Boston, MA
Background/Purpose: Serostatus may be associated with different responses to treatment with biologic disease-modifying anti-rheumatic drugs (DMARD) or Janus kinase inhibitors (JAKi) in patients with rheumatoid arthritis (RA). To date, few studies examined the comparative effectiveness of RA treatment specific to serostatus using large real-world data. Therefore, we aimed to evaluate the clinical effectiveness of biologic DMARDs or JAKi among seropositive RA patients compared with seronegative patients in a real-world setting using claims-based surrogate endpoints of clinical effectiveness.
Methods: We used Optum Clinformatics® Data Mart (01/01/2004 - 03/31/2021) linked with outpatient laboratory test results. The study population was biologic/JAKi naïve patients who initiated one of the following drugs: tumor necrosis factor-alpha inhibitors (TNFi, i.e., adalimumab, certolizumab pegol, etanercept, golimumab, infliximab), abatacept, interleukin (IL)-6 inhibitors (i.e., sarilumab, tocilizumab), or JAKi, (i.e., baricitinib, tofacitinib, upadacitinib). Dispensing of the first-ever biologic DMARD or JAKi was defined as the index date. Eligible subjects were required to 1) have ≥2 RA diagnostic codes separated by 7-365 days before the index date, 2) have ≥365 days of continuous enrollment within 1 year before and after the index date, and 3) be ≥18 years old. To assess patients' serostatus, we further restricted to patients who had rheumatoid factor (RF) or anticitrullinated protein antibody (ACPA) results or International Classification of Diseases Tenth Version (ICD-10) code of M05*/M06.0* any time prior to the index date. We assessed clinical effectiveness based on a composite endpoint of claims -based treatment failure at 1 year after treatment initiation: 1) discontinuation of the index drug, 2) switching to another biologic or JAK inhibitor, 3) switching the index csDMARD, 4) initiation of an oral steroid, 5) increased dose of oral steroid, or 6) death. Log-binomial regression models were constructed to estimate the crude or adjusted risk ratio (RR) with 95% confidence interval (CI) adjusting for over 60 covariates including index DMARD, interaction term of index DMARD group and serostatus, demographics, baseline comorbidities, comedications healthcare utilization.
Results: We identified a total of 12,045 RA patients: 7,824 seropositive and 4,221 seronegative. There were 81.1% TNFi, 8.2% abatacept, 3.4% IL-6 inhibitor, and 7.2% JAKi initiators (Table 1). Mean (SD) age was 56.7 (14.0) years old, and 77.9% were female. Seropositive patients were older and had a higher comorbidity score than seronegative patients. The risk of 1-year treatment failure was 29.8% among seropositive and 30.2% among seronegative patients (Table 2). The crude and adjusted RR (95% CI) were 0.99 (0.93, 1.04) and 1.01 (0.95, 1.09), respectively. We did not observe a difference in the risk of treatment failure for seropositive vs. seronegative RA patients across the different DMARDs (p-value for interaction term = 0.945).
Conclusion: In this real-world observational study of patients with RA, seropositive and seronegative RA patients had similar clinical effectiveness 1 year after newly starting a biologic DMARD or JAKi.
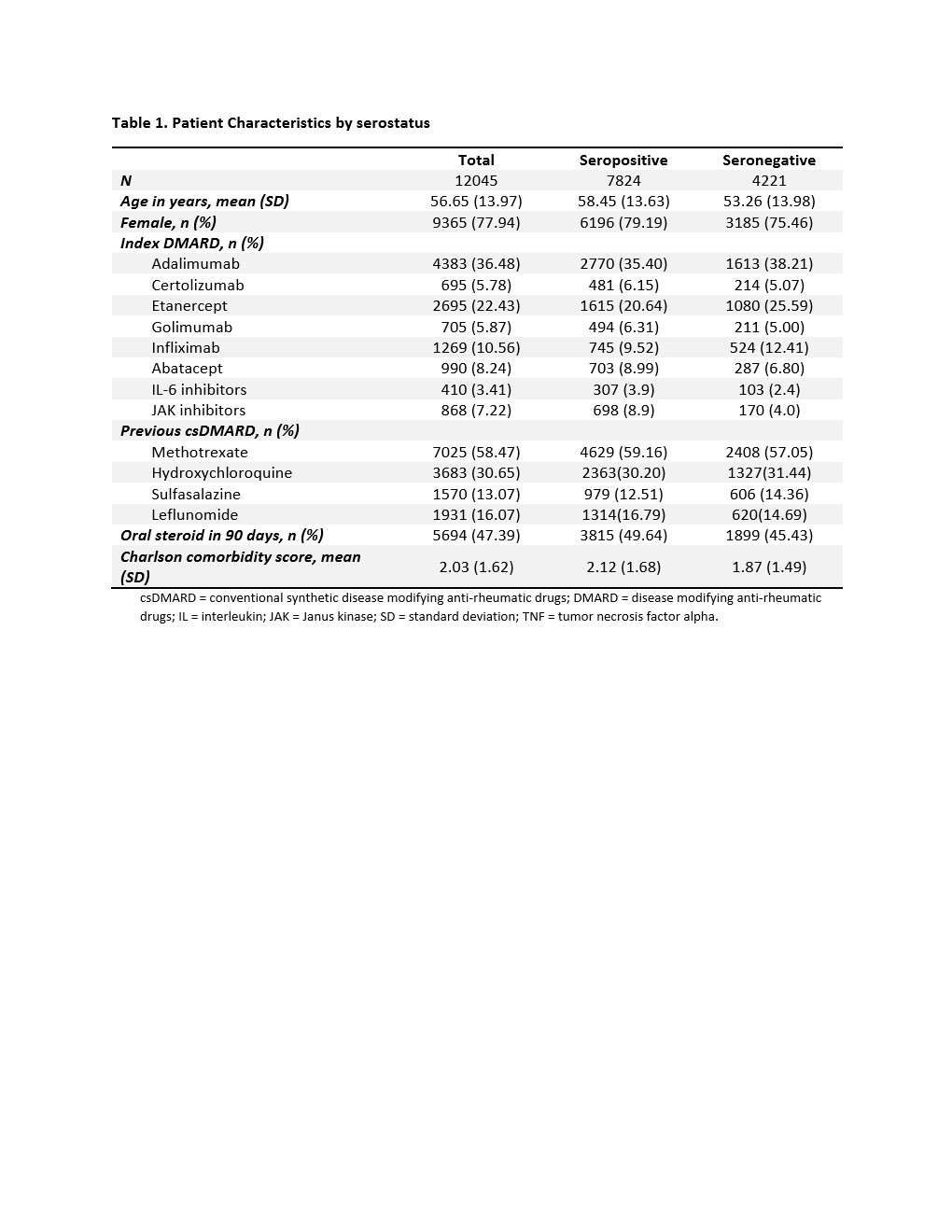 Table 1. Patient Characteristics by serostatus
Table 1. Patient Characteristics by serostatus
csDMARD = conventional synthetic disease modifying anti-rheumatic drugs; DMARD = disease modifying anti-rheumatic drugs; IL = interleukin; JAK = Janus kinase; SD = standard deviation; TNF = tumor necrosis factor alpha.
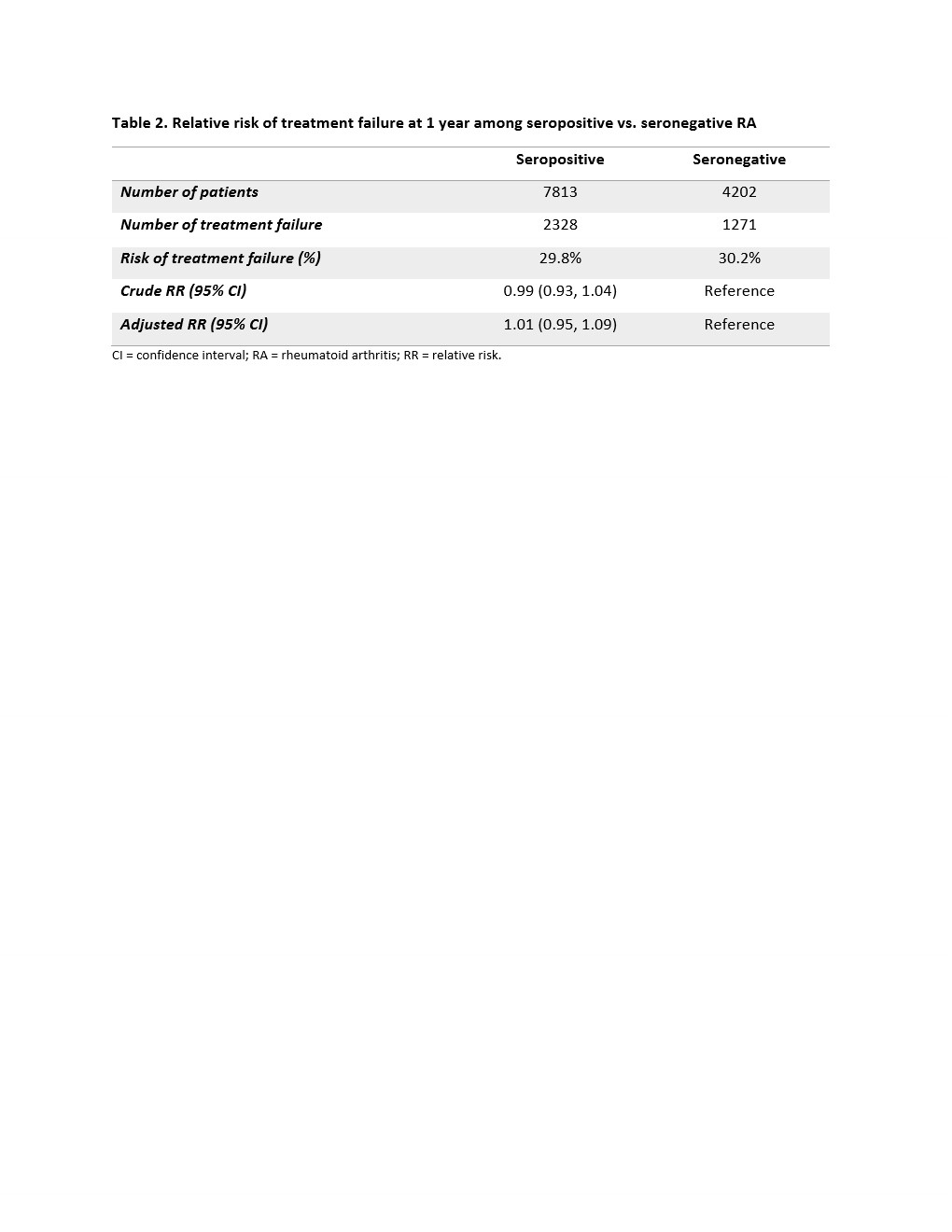 Table 2. Relative risk of treatment failure at 1 year among seropositive vs. seronegative RA
Table 2. Relative risk of treatment failure at 1 year among seropositive vs. seronegative RA
CI = confidence interval; RA = rheumatoid arthritis; RR = relative risk.
Disclosures: Y. Jin, None; J. Liu, None; R. Desai, Bayer, Vertex, Novartis; S. Kim, Pfizer, AbbVie/Abbott, Roche, Bristol-Myers Squibb(BMS).
Background/Purpose: Serostatus may be associated with different responses to treatment with biologic disease-modifying anti-rheumatic drugs (DMARD) or Janus kinase inhibitors (JAKi) in patients with rheumatoid arthritis (RA). To date, few studies examined the comparative effectiveness of RA treatment specific to serostatus using large real-world data. Therefore, we aimed to evaluate the clinical effectiveness of biologic DMARDs or JAKi among seropositive RA patients compared with seronegative patients in a real-world setting using claims-based surrogate endpoints of clinical effectiveness.
Methods: We used Optum Clinformatics® Data Mart (01/01/2004 - 03/31/2021) linked with outpatient laboratory test results. The study population was biologic/JAKi naïve patients who initiated one of the following drugs: tumor necrosis factor-alpha inhibitors (TNFi, i.e., adalimumab, certolizumab pegol, etanercept, golimumab, infliximab), abatacept, interleukin (IL)-6 inhibitors (i.e., sarilumab, tocilizumab), or JAKi, (i.e., baricitinib, tofacitinib, upadacitinib). Dispensing of the first-ever biologic DMARD or JAKi was defined as the index date. Eligible subjects were required to 1) have ≥2 RA diagnostic codes separated by 7-365 days before the index date, 2) have ≥365 days of continuous enrollment within 1 year before and after the index date, and 3) be ≥18 years old. To assess patients' serostatus, we further restricted to patients who had rheumatoid factor (RF) or anticitrullinated protein antibody (ACPA) results or International Classification of Diseases Tenth Version (ICD-10) code of M05*/M06.0* any time prior to the index date. We assessed clinical effectiveness based on a composite endpoint of claims -based treatment failure at 1 year after treatment initiation: 1) discontinuation of the index drug, 2) switching to another biologic or JAK inhibitor, 3) switching the index csDMARD, 4) initiation of an oral steroid, 5) increased dose of oral steroid, or 6) death. Log-binomial regression models were constructed to estimate the crude or adjusted risk ratio (RR) with 95% confidence interval (CI) adjusting for over 60 covariates including index DMARD, interaction term of index DMARD group and serostatus, demographics, baseline comorbidities, comedications healthcare utilization.
Results: We identified a total of 12,045 RA patients: 7,824 seropositive and 4,221 seronegative. There were 81.1% TNFi, 8.2% abatacept, 3.4% IL-6 inhibitor, and 7.2% JAKi initiators (Table 1). Mean (SD) age was 56.7 (14.0) years old, and 77.9% were female. Seropositive patients were older and had a higher comorbidity score than seronegative patients. The risk of 1-year treatment failure was 29.8% among seropositive and 30.2% among seronegative patients (Table 2). The crude and adjusted RR (95% CI) were 0.99 (0.93, 1.04) and 1.01 (0.95, 1.09), respectively. We did not observe a difference in the risk of treatment failure for seropositive vs. seronegative RA patients across the different DMARDs (p-value for interaction term = 0.945).
Conclusion: In this real-world observational study of patients with RA, seropositive and seronegative RA patients had similar clinical effectiveness 1 year after newly starting a biologic DMARD or JAKi.
 Table 1. Patient Characteristics by serostatus
Table 1. Patient Characteristics by serostatuscsDMARD = conventional synthetic disease modifying anti-rheumatic drugs; DMARD = disease modifying anti-rheumatic drugs; IL = interleukin; JAK = Janus kinase; SD = standard deviation; TNF = tumor necrosis factor alpha.
 Table 2. Relative risk of treatment failure at 1 year among seropositive vs. seronegative RA
Table 2. Relative risk of treatment failure at 1 year among seropositive vs. seronegative RACI = confidence interval; RA = rheumatoid arthritis; RR = relative risk.
Disclosures: Y. Jin, None; J. Liu, None; R. Desai, Bayer, Vertex, Novartis; S. Kim, Pfizer, AbbVie/Abbott, Roche, Bristol-Myers Squibb(BMS).

