Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0629–0670) SLE – Etiology and Pathogenesis Poster
0637: Reduced IgG Sialic Acid Content: A Distinctive Characteristic of Symptomatic Anti-Nuclear Antibodies Positive Individuals
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- CM
Carolina Munoz-Grajales, MD
UHN/TWH
Toronto, ON, Canada
Abstract Poster Presenter(s)
Carolina Munoz1, Sindu Johnson2, Zahi Touma1, Zareen Ahmad2, Dennisse Bonilla1, Linda Hiraki3, Arthur Bookman2 and Joan Wither4, 1Schroeder Arthritis Institute, Krembil Research Institute, University Health Network and University of Toronto, Toronto, ON, Canada, 2University of Toronto, Toronto, ON, Canada, 3The Hospital for Sick Children, Division of Rheumatology, Department of Paediatrics, University of Toronto, Genetics and Genome Biology, SickKids Research Institute, Toronto, ON, Canada, 4Schroeder Arthritis Institute, Krembil Research Institute, University Health Network, University of Toronto, Toronto, ON, Canada
Background/Purpose: Currently the immune changes that lead to the transition from asymptomatic Anti-Nuclear Antibody (ANA) positivity to symptomatic disease are unknown. Studies in our laboratory revealed that increased levels of proinflammatory factors, particularly TNF-a, are restricted to symptomatic ANA+ individuals. Based upon this observation and the similarities between the ANA-associated SARDs (Systemic Lupus Erythematosus (SLE), Sjogren's Syndrome, and Systemic Sclerosis) and Rheumatoid Arthritis, where progression from asymptomatic to symptomatic autoimmunity is associated with reduced sialylation of the IgG Fc region and accumulation of pro-inflammatory cytokines, herein we proposed to investigate whether or not a similar shift in sialylation occurs as asymptomatic ANA+ individuals progress to SARD.
Methods: An enzyme-linked lectin assay was developed that uses Sambucus Nigra Agglutinin, a lectin that binds sialic acid, to detect sialylated IgG. This assay was then used to determine the extent of sialylation on IgG purified from the plasma of 10 ANA- healthy controls (ANA-HC), 15 ANA+ asymptomatic (ANA+NS) individuals, and 15 SLE patients. Differences is the ability of the IgG to elicit inflammation between the ANA+ groups were investigated by stimulating monocyte derived dendric cells (moDC) from ANA-HC with aggregated IgG. IL-6 and TNF-a in the culture supernatants and serum were measured by ELISA. Cellular profiling of peripheral blood immune populations was performed using flow cytometry. Differences between the 3 groups were determined using the Kruskal-Wallis test followed by Dunn's post-test for multiple comparisons. Correlations were assessed using Spearman's correlation coefficient.
Results: The sialic acid content of IgG was significantly reduced in SLE patients compared to ANA+NS individuals (p= 0.02) and ANA-HC (p= 0.001) (Figure 1A). When moDC were stimulated with heat-aggregated IgG from ANA+NS and SLE patients, there was a trend to increased production of IL-6 and TNF-a, as compared to heat-aggregated IgG from ANA-HC, which was statically significant for TNF-a (p= 0.008) (Figure 1B) in SLE. There was a negative correlation between sialylation of IgG and serum levels of TNF-a (rho= -0.54, p= 0.004) (Figure 1C). The extent of IgG sialylation demonstrated a negative correlation with the levels of TNF-a produced in response to heat-aggregated IgG (rho= -0.5, p=0.02) (Figure 1D) and the proportion of T follicular 17 helper cells (rho= -0.37, p= 0.03) in the patients from which the IgG was purified (Figure 1E).
Conclusion: The reduced levels of sialylated IgG and their association with increased levels of TNF-a production in SLE patients, as compared to ANA+NS individuals and ANA-HC, are compatible with the concept that de-sialylation of IgG promotes the transition from asymptomatic to symptomatic autoimmunity in SARD.
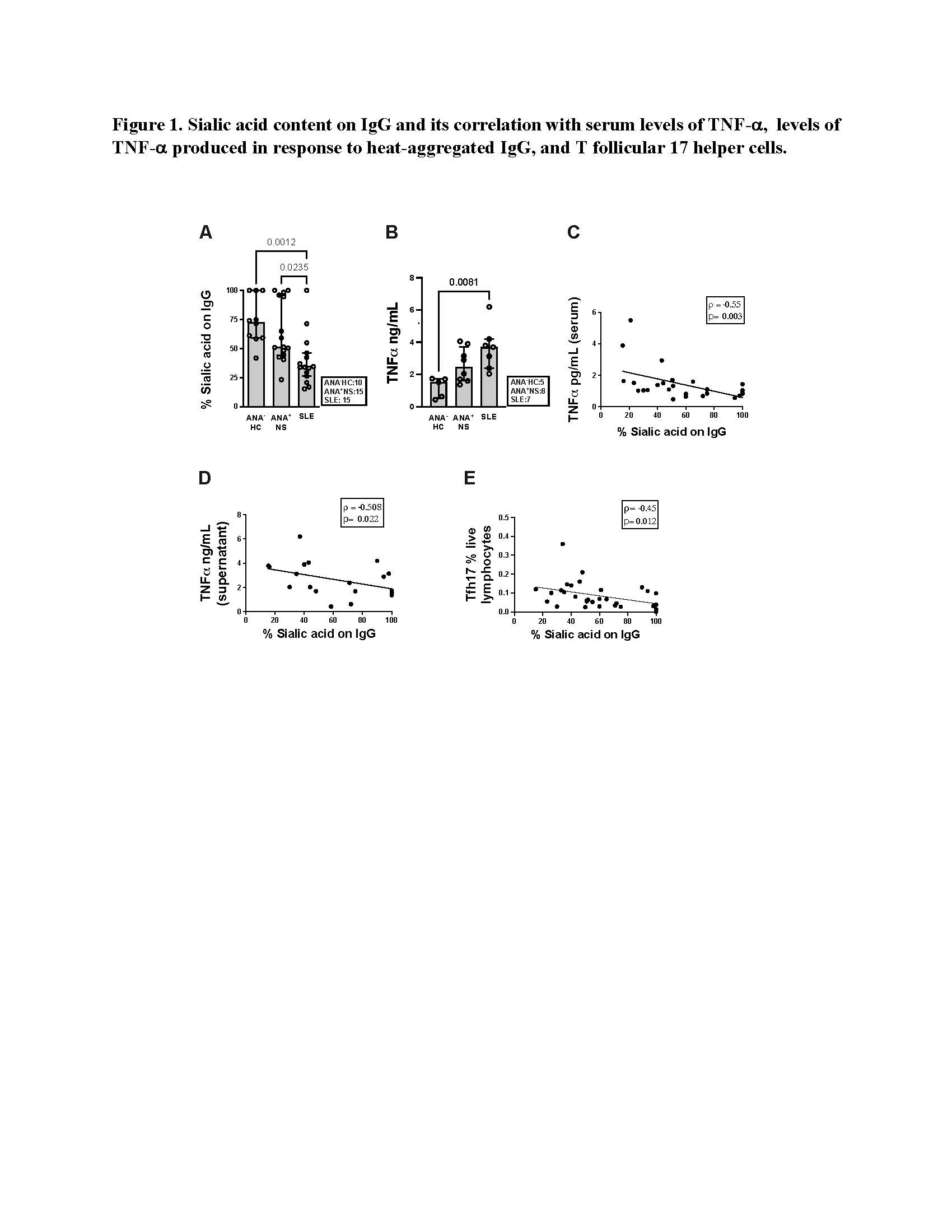 Figure 1. Sialic acid content on IgG and its correlation with serum levels of TNF-, levels of TNF- produced in response to heat-aggregated IgG, and T follicular 17 helper cells.
Figure 1. Sialic acid content on IgG and its correlation with serum levels of TNF-, levels of TNF- produced in response to heat-aggregated IgG, and T follicular 17 helper cells.
Disclosures: C. Munoz, None; S. Johnson, None; Z. Touma, None; Z. Ahmad, None; D. Bonilla, None; L. Hiraki, None; A. Bookman, None; J. Wither, AstraZeneca, Pfizer.
Background/Purpose: Currently the immune changes that lead to the transition from asymptomatic Anti-Nuclear Antibody (ANA) positivity to symptomatic disease are unknown. Studies in our laboratory revealed that increased levels of proinflammatory factors, particularly TNF-a, are restricted to symptomatic ANA+ individuals. Based upon this observation and the similarities between the ANA-associated SARDs (Systemic Lupus Erythematosus (SLE), Sjogren's Syndrome, and Systemic Sclerosis) and Rheumatoid Arthritis, where progression from asymptomatic to symptomatic autoimmunity is associated with reduced sialylation of the IgG Fc region and accumulation of pro-inflammatory cytokines, herein we proposed to investigate whether or not a similar shift in sialylation occurs as asymptomatic ANA+ individuals progress to SARD.
Methods: An enzyme-linked lectin assay was developed that uses Sambucus Nigra Agglutinin, a lectin that binds sialic acid, to detect sialylated IgG. This assay was then used to determine the extent of sialylation on IgG purified from the plasma of 10 ANA- healthy controls (ANA-HC), 15 ANA+ asymptomatic (ANA+NS) individuals, and 15 SLE patients. Differences is the ability of the IgG to elicit inflammation between the ANA+ groups were investigated by stimulating monocyte derived dendric cells (moDC) from ANA-HC with aggregated IgG. IL-6 and TNF-a in the culture supernatants and serum were measured by ELISA. Cellular profiling of peripheral blood immune populations was performed using flow cytometry. Differences between the 3 groups were determined using the Kruskal-Wallis test followed by Dunn's post-test for multiple comparisons. Correlations were assessed using Spearman's correlation coefficient.
Results: The sialic acid content of IgG was significantly reduced in SLE patients compared to ANA+NS individuals (p= 0.02) and ANA-HC (p= 0.001) (Figure 1A). When moDC were stimulated with heat-aggregated IgG from ANA+NS and SLE patients, there was a trend to increased production of IL-6 and TNF-a, as compared to heat-aggregated IgG from ANA-HC, which was statically significant for TNF-a (p= 0.008) (Figure 1B) in SLE. There was a negative correlation between sialylation of IgG and serum levels of TNF-a (rho= -0.54, p= 0.004) (Figure 1C). The extent of IgG sialylation demonstrated a negative correlation with the levels of TNF-a produced in response to heat-aggregated IgG (rho= -0.5, p=0.02) (Figure 1D) and the proportion of T follicular 17 helper cells (rho= -0.37, p= 0.03) in the patients from which the IgG was purified (Figure 1E).
Conclusion: The reduced levels of sialylated IgG and their association with increased levels of TNF-a production in SLE patients, as compared to ANA+NS individuals and ANA-HC, are compatible with the concept that de-sialylation of IgG promotes the transition from asymptomatic to symptomatic autoimmunity in SARD.
 Figure 1. Sialic acid content on IgG and its correlation with serum levels of TNF-, levels of TNF- produced in response to heat-aggregated IgG, and T follicular 17 helper cells.
Figure 1. Sialic acid content on IgG and its correlation with serum levels of TNF-, levels of TNF- produced in response to heat-aggregated IgG, and T follicular 17 helper cells.Disclosures: C. Munoz, None; S. Johnson, None; Z. Touma, None; Z. Ahmad, None; D. Bonilla, None; L. Hiraki, None; A. Bookman, None; J. Wither, AstraZeneca, Pfizer.

