Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0974–1003) SLE – Treatment Poster II
0989: Regulatory T Cell Defects in SLE and Therapy with a Novel IL-2 Mutein: Phase 1 Clinical Results with Efavaleukin Alfa
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- NS
Nandita Sarkar, PhD
Amgen
South San Francisco, CA, United States
Abstract Poster Presenter(s)
Nandita Sarkar1, Xuguang Hu1, Nadia Tchao1, Richard Furie2, Alan Kivitz3, Stanley B Cohen4 and Kevin Gorski5, 1Amgen Inc., South San Francisco, CA, 2Northwell Health, Great Neck, NY, 3Department of Rheumatology, Altoona Center for Clinical Research, Duncansville, PA, 4Metroplex Clinical Research Center and University of Texas Southwestern Medical Center, Dallas, TX, 5Amgen, Inc., Thousand Oaks, CA
Background/Purpose: Regulatory T cells (Treg) are critical for maintaining self-tolerance and preventing autoimmunity, and IL-2 is essential for their development, survival and suppressive function. Defects in Treg function and the IL-2 receptor are associated with SLE and have been correlated with disease activity (von Spee-Mayer 2016; Bonelli M 2009). Efavaleukin alfa is an IL-2 mutein Fc fusion protein that preferentially binds the high-affinity IL-2 receptor alpha chain (CD25) to selectively promote Treg expansion. We compared Treg functional phenotypes in healthy controls (phase 1a baseline) to SLE patients (at baseline and post-treatment) enrolled in a phase 1b study of efavaleukin alfa to characterize Treg defects in SLE and the effects of IL-2-targeted therapy with efavaleukin alfa on Treg expansion.
Methods: Baseline Treg functional phenotyping data from 64 healthy volunteers (ph1a baseline) were compared with Treg data from phase 1b SLE study subjects. The phase 1b, multiple ascending dose study (NCT03451422) included 5 dose cohorts; a total of 35 patients with SLE (with either elevated anti-dsDNA or ANA) were randomized to receive efavaleukin alfa or placebo SC every 2 weeks (Q2W; cohorts 1, 2, 4, and 5) or every week (QW; cohort 3) for a total of 12 weeks. Pharmacodynamic profiles of lymphocyte subsets were evaluated in peripheral blood. Cell subsets were defined as follows: Treg, CD3+CD4+Foxp3+CD127–; CD25bright Treg, Treg with the highest CD25 expression; CD25– Treg, CD3+CD4+Foxp3+CD127–CD25–; and CD4+ Tcon, CD3+CD4+(-Treg).
Results: At baseline, Treg CD25 expression levels were significantly lower in SLE patients compared with healthy subjects (Fig 1a), and CD25 expression on Foxp3+ Treg negatively correlated with anti-dsDNA autoantibodies (Fig 1b). The percentage of CD25– Tregs was significantly higher in SLE patients than healthy subjects and also correlated with anti-dsDNA at baseline. At day 22 in the phase 1b study, after SLE patients randomized to efavaleukin alfa had received 2 doses, Treg CD25 expression was significantly increased above baseline and comparable to the levels in healthy controls (Fig 1a). The number of CD25bright Tregs in SLE patients was inversely correlated with anti-dsDNA at baseline, with a mean peak increase of 53.8-fold above baseline with efavaleukin alfa treatment. The intensity of CD25 on CD4+ Tcon was not significantly different between healthy subjects and SLE patients and was not increased with efavaleukin alfa treatment.
Conclusion: Treg from SLE patients showed defects in IL-2 signaling evidenced by lower CD25 expression that correlated with anti-dsDNA, suggesting a role for reduced IL-2 signaling in SLE autoimmunity. Efavaleukin alfa treatment selectively restored Treg CD25 expression to levels observed in healthy controls without increasing CD25 expression on Tcon. CD25bright Treg, considered a more suppressive Treg population, were expanded with multiple doses of efavaleukin alfa. Although low baseline SLE disease activity and short treatment duration precluded efficacy analysis, clinical correlations with treatment response will be investigated in the ongoing phase 2b adaptive trial of efavaleukin alfa in SLE.
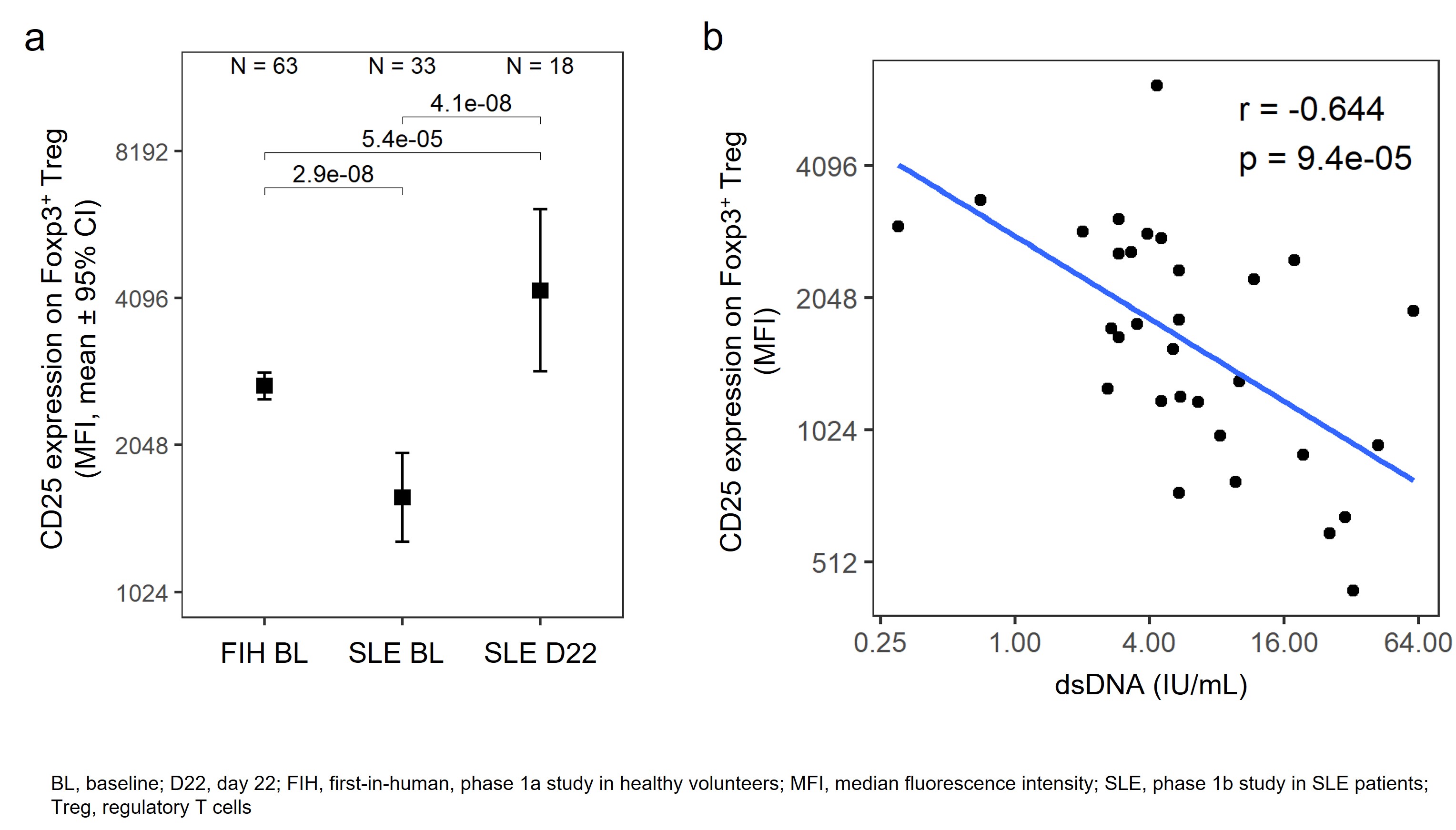 Figure: CD25 expression levels on Treg at baseline and with efavaleukin alfa treatment (a) and the baseline correlation to anti-dsDNA antibodies (b)
Figure: CD25 expression levels on Treg at baseline and with efavaleukin alfa treatment (a) and the baseline correlation to anti-dsDNA antibodies (b)
Disclosures: N. Sarkar, Amgen; X. Hu, Amgen Inc; N. Tchao, Amgen Inc; R. Furie, AstraZeneca, Biogen; A. Kivitz, Amgen, Boehringer-Ingelheim, Janssen, Gilead, GlaxoSmithKlein (GSK), Novartis, Pfizer, Sanofi, Flexion, Eli Lilly, Genentech, UCB, AbbVie, Merck, ECOR1 CAPITAL, LLC, Chemocentryx, Regenerson, Grunenthal, Bendcare, Horizon; S. Cohen, Amgen, AbbVie, Eli Lilly, Pfizer Inc, Gilead; K. Gorski, Amgen Inc.
Background/Purpose: Regulatory T cells (Treg) are critical for maintaining self-tolerance and preventing autoimmunity, and IL-2 is essential for their development, survival and suppressive function. Defects in Treg function and the IL-2 receptor are associated with SLE and have been correlated with disease activity (von Spee-Mayer 2016; Bonelli M 2009). Efavaleukin alfa is an IL-2 mutein Fc fusion protein that preferentially binds the high-affinity IL-2 receptor alpha chain (CD25) to selectively promote Treg expansion. We compared Treg functional phenotypes in healthy controls (phase 1a baseline) to SLE patients (at baseline and post-treatment) enrolled in a phase 1b study of efavaleukin alfa to characterize Treg defects in SLE and the effects of IL-2-targeted therapy with efavaleukin alfa on Treg expansion.
Methods: Baseline Treg functional phenotyping data from 64 healthy volunteers (ph1a baseline) were compared with Treg data from phase 1b SLE study subjects. The phase 1b, multiple ascending dose study (NCT03451422) included 5 dose cohorts; a total of 35 patients with SLE (with either elevated anti-dsDNA or ANA) were randomized to receive efavaleukin alfa or placebo SC every 2 weeks (Q2W; cohorts 1, 2, 4, and 5) or every week (QW; cohort 3) for a total of 12 weeks. Pharmacodynamic profiles of lymphocyte subsets were evaluated in peripheral blood. Cell subsets were defined as follows: Treg, CD3+CD4+Foxp3+CD127–; CD25bright Treg, Treg with the highest CD25 expression; CD25– Treg, CD3+CD4+Foxp3+CD127–CD25–; and CD4+ Tcon, CD3+CD4+(-Treg).
Results: At baseline, Treg CD25 expression levels were significantly lower in SLE patients compared with healthy subjects (Fig 1a), and CD25 expression on Foxp3+ Treg negatively correlated with anti-dsDNA autoantibodies (Fig 1b). The percentage of CD25– Tregs was significantly higher in SLE patients than healthy subjects and also correlated with anti-dsDNA at baseline. At day 22 in the phase 1b study, after SLE patients randomized to efavaleukin alfa had received 2 doses, Treg CD25 expression was significantly increased above baseline and comparable to the levels in healthy controls (Fig 1a). The number of CD25bright Tregs in SLE patients was inversely correlated with anti-dsDNA at baseline, with a mean peak increase of 53.8-fold above baseline with efavaleukin alfa treatment. The intensity of CD25 on CD4+ Tcon was not significantly different between healthy subjects and SLE patients and was not increased with efavaleukin alfa treatment.
Conclusion: Treg from SLE patients showed defects in IL-2 signaling evidenced by lower CD25 expression that correlated with anti-dsDNA, suggesting a role for reduced IL-2 signaling in SLE autoimmunity. Efavaleukin alfa treatment selectively restored Treg CD25 expression to levels observed in healthy controls without increasing CD25 expression on Tcon. CD25bright Treg, considered a more suppressive Treg population, were expanded with multiple doses of efavaleukin alfa. Although low baseline SLE disease activity and short treatment duration precluded efficacy analysis, clinical correlations with treatment response will be investigated in the ongoing phase 2b adaptive trial of efavaleukin alfa in SLE.
 Figure: CD25 expression levels on Treg at baseline and with efavaleukin alfa treatment (a) and the baseline correlation to anti-dsDNA antibodies (b)
Figure: CD25 expression levels on Treg at baseline and with efavaleukin alfa treatment (a) and the baseline correlation to anti-dsDNA antibodies (b)Disclosures: N. Sarkar, Amgen; X. Hu, Amgen Inc; N. Tchao, Amgen Inc; R. Furie, AstraZeneca, Biogen; A. Kivitz, Amgen, Boehringer-Ingelheim, Janssen, Gilead, GlaxoSmithKlein (GSK), Novartis, Pfizer, Sanofi, Flexion, Eli Lilly, Genentech, UCB, AbbVie, Merck, ECOR1 CAPITAL, LLC, Chemocentryx, Regenerson, Grunenthal, Bendcare, Horizon; S. Cohen, Amgen, AbbVie, Eli Lilly, Pfizer Inc, Gilead; K. Gorski, Amgen Inc.

