Back
Abstract Session
Session: Abstracts: Infection-related Rheumatic Disease (2272–2275)
2273: Waning Vaccine Response After Primary Vaccine Series: Results from the Covid19 Vaccine Response in Rheumatology Patients (COVER) Study
Monday, November 14, 2022
5:15 PM – 5:25 PM Eastern Time
Location: Exhibit Hall A
- AM
Amy Mudano, MPH
University of Alabama at BIrmingham
Birmingham, Alabama, United States
Presenting Author(s)
Amy Mudano1, Gary Cutter2, Ted Mikuls3, Geoffrey Thiele4, Mark Law4, Bart Hamilton4, Michael Zikry5, Kelly Y. Chun6, Monique Bastidas7, Michael George8, Lisa Williams9, Kevin Winthrop10, Mark Busch11, Stanley Cohen12, Roman Czubatyj13, Rajesh Kataria14, Reshma Khan15, Soha Mousa16, Jose Pando17, Elizabeth Perkins18, Shanmugapriya Reddy19, Delfin Santos20, Joy Schechtman21, Frank Scott22, Sucharitha Shanmugam23, Atul Singhal24, John Tesser25, John Tower26, Swamy Venuturupalli27, Sean Joseph Wollaston28, Conrad Ziembinski29 and Jeffrey Curtis30, 1Illumination Health, Hoover, AL, 2University of Alabama at Birmingham, Birmingham, AL, 3Division of Rheumatology, University of Nebraska Medical Center, Omaha, NE, 4University of Nebraska Medical Center, Omaha, NE, 5LabCorp, Burlington, NC, 6Labcorp, Calabasas, CA, 7LabCorp, Northridge, CA, 8University of Pennsylvania, Philadelphia, PA, 9Illumination Heath, Hoover, AL, 10Oregon Health & Science University, Portland, OR, 11Family Arthritis Center-Jupiter, Jupiter, FL, 12Metroplex Clinical Research Center, Pittsburgh, PA, 13Arthritis Physicians LLC, Rochester Hills, MI, 14Southern Ohio, Wheelersburg, OH, 15Palm Beach Rheumatology and Wellness, Jupiter, FL, 16Arthritis and Rheumatology of Southwest Ohio, Liberty Township, OH, 17Rheumatology Consultants of DE, Lewes, DE, 18Rheumatology Care Center, Hoover, AL, 19Southwest Florida Rheumatology, Riverview, FL, 20Rochester Rheumatology, Rochester Hills, MI, 21SunValley Arthritis Center, Peoria, AZ, 22Arthritis Medical Center, Arroyo Grande, CA, 23Rheumatology and Arthritis Care Center, Exton, PA, 24Southwest Rheumatology Research, Mesquite, TX, 25Arizona Arthritis & Rheumatology Associates, Phoenix, AZ, 26Arthritis Physicians LLC, Rochester, MI, 27Cedars-Sinai Medical Center, Los Angeles, CA, 28Rheumatology Associates, North Hollywood, CA, 29CZ Rheumatology, Coral Springs, FL, 30Division of Clinical Immunology & Rheumatology, University of Alabama at Birmingham, Birmingham, AL
Background/Purpose: Given possible increased risk of COVID-19 in patients with autoimmune conditions, there is a need to better understand the immunogenicity and safety of SARS-CoV-2 vaccines in people living with rheumatic disease. We are currently conducting the Covid VaccinE Response (COVER) trial, a multicenter, randomized controlled trial to evaluate the response to a SARS-CoV-2 booster in patients with confirmed rheumatic disease on immunomodulatory therapies. In this analysis, we examined response to the primary mRNA vaccine series in relation to the time since vaccination, and therapy.
Methods: COVER randomizes rheumatoid arthritis (RA) or spondyloarthritis (SpA) patients in a large practice-based research network of U.S. rheumatologists to continue their current immunomodulatory therapy regimen or briefly interrupt (ie, hold for 2 weeks) following administration of a 3rd or 4th dose (booster) of SARS-CoV-2 vaccine. In this analysis, baseline vaccine response was examined cross-sectionally in relation to time since the primary vaccine series. LabCorp Cov2Quant IgG™ Spike assay was used to measure levels of anti-Receptor Binding Domain (RBD) IgG antibodies in response to vaccination and or/natural infection. Anti-Nucleocapsid antibodies were measured to identify prior SARS-CoV-2 infection and evidence of prior COVID-19 infection was also classified using self-report. Demographic characteristics were reported, and COVID-19 RBD antibody levels examined in relation to time since primary vaccination. Linear regression was used to evaluate determinants of mean log anti-RBD antibody titers and compared by drug categories.
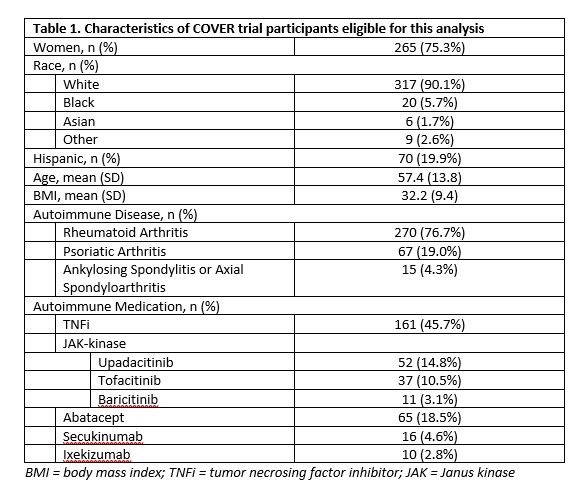
Results: A total of 352 participants completed a baseline visit and were eligible for analysis (Table 1). Participants were 75% women, 90% white, 20% Hispanic. Mean age: 57.4 (13.8), mean BMI 32.2 (9.4). The majority had RA (77%); 46% TNFi use, 28% JAKi, 27% others (abatacept, IL-17i) at primary vaccine series. Among those with no prior COVID-19 infection, anti-RBD IgG antibody titers showed a clear decrease with time since receipt of prior vaccination (Figure 1). In adjusted linear regression, referent to TNFi, JAKi (p=0.02) and abatacept (p=0.05) patients had lower mean titers, even after accounting for time since vaccine, number of vaccine doses and Anti-Nucleocapsid status. Table 2 shows the comparison of anti-Nucleocapsid status at baseline with participant self-report of prior COVID-19 infection, specificity was 78% (95% CI: 72%-83%) and sensitivity was 65% (55%-73%).
Conclusion: A clear decrease of anti-RBD IgG antibody levels in patients with RA or SpA taking immunomodulatory therapy was seen with increasing time following previous vaccine receipt. This effect was more pronounced in those who reported no prior COVID-19 infection, and who were receiving JAKi or abatacept (referent to TNFi). Self-report of prior COVID infection substantially misclassified prior COVID-19 infection and may confound immunogenicity outcomes in vaccine studies. Future analysis of the randomized trial results will look at the effect of holding or continuing immunomodulatory treatment at the time a SARS-CoV-2 booster is given.
.jpg)

Disclosures: A. Mudano, None; G. Cutter, AI Therapeutics, AMO Pharma., Astra-Zeneca,, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring,, Galmed Pharmaceuticals, Green Valley Pharma, Horizon Pharmaceuticals, mmunic, Mapi Pharmaceuticals LTD, Merck, Mitsubishi Tanabe Pharma Holdings, Opko Biologics, Prothena Biosciences, Novartis, Regeneron,, Sanofi-Aventis, Reata Pharmaceuticals, Alexion, Antisense Therapeutics, Biogen, Clinical Trial Solutions LLC, Genzyme, Genentech, GW Pharmaceuticals/Jazz, Klein-Buendel Incorporated, Merck/Serono, Osmotica Pharmaceuticals, Perception Neurosciences, Protalix Biotherapeutics,, Recursion/Cerexis Pharmaceuticals, Roche, SAB Biotherapeutics; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; G. Thiele, None; M. Law, None; B. Hamilton, None; M. Zikry, Labcorp, labcorp; K. Chun, None; M. Bastidas, LabCorp; M. George, AbbVie, GlaxoSmithKlein(GSK), Chemocentryx; L. Williams, None; K. Winthrop, AbbVie, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GSK, Pfizer, Roche, Regeneron, Sanofi, UCB, AstraZeneca, Novartis; M. Busch, Amgen, AbbVie/Abbott, GlaxoSmithKlein(GSK), UCB, horizon; S. Cohen, AbbVie/Abbott, Amgen, Gilead, Eli Lilly, Pfizer; R. Czubatyj, AbbVie/Abbott, Amgen, Horizon, Exagen, Janssen; R. Kataria, AbbVie/Abbott, Amgen, Horizon, Scipher; R. Khan, Amgen, Janssen, GlaxoSmithKlein(GSK); S. Mousa, None; J. Pando, AbbVie/Abbott, Novartis; E. Perkins, AbbVie/Abbott, Novartis, Janssen; S. Reddy, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Janssen, Novartis, Pfizer, UCB; D. Santos, Pfizer, Amgen, AbbVie/Abbott; J. Schechtman, Exagen, Mallinckrodt, Horizon, Pfizer, UCB, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Lilly, Myriad, Boehringer-Ingelheim, Genentech, Scipher, Janssen, Radius, Amgen, ChemoCentryx; F. Scott, None; S. Shanmugam, AstraZeneca; A. Singhal, None; J. Tesser, AbbVie, Amgen, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, GlaxoSmithKlein (GSK), Janssen, Gilead, Merck/MSD, Novartis, Pfizer, UCB, Aurinia, Genentech, Sanofi-Genzyme, Biogen, Celgene, CSL Behring, Horizon, R-Pharma, Regeneron, Roche, Sandoz, Scipher, Takeda, Sun Pharma, Selecta, Vorso; J. Tower, Amgen; S. Venuturupalli, Horizon Pharma USA, Inc., Kezar Life Sciences, Inc., Mallinckrodt, Inc., Navidea Biopharmaceuticals, Inc., Pfizer (Current), Bristol-Myers Squibb(BMS), Argenx, Janssen; S. Wollaston, None; C. Ziembinski, UCB, Scipher, CorEvitas; J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower.
Background/Purpose: Given possible increased risk of COVID-19 in patients with autoimmune conditions, there is a need to better understand the immunogenicity and safety of SARS-CoV-2 vaccines in people living with rheumatic disease. We are currently conducting the Covid VaccinE Response (COVER) trial, a multicenter, randomized controlled trial to evaluate the response to a SARS-CoV-2 booster in patients with confirmed rheumatic disease on immunomodulatory therapies. In this analysis, we examined response to the primary mRNA vaccine series in relation to the time since vaccination, and therapy.
Methods: COVER randomizes rheumatoid arthritis (RA) or spondyloarthritis (SpA) patients in a large practice-based research network of U.S. rheumatologists to continue their current immunomodulatory therapy regimen or briefly interrupt (ie, hold for 2 weeks) following administration of a 3rd or 4th dose (booster) of SARS-CoV-2 vaccine. In this analysis, baseline vaccine response was examined cross-sectionally in relation to time since the primary vaccine series. LabCorp Cov2Quant IgG™ Spike assay was used to measure levels of anti-Receptor Binding Domain (RBD) IgG antibodies in response to vaccination and or/natural infection. Anti-Nucleocapsid antibodies were measured to identify prior SARS-CoV-2 infection and evidence of prior COVID-19 infection was also classified using self-report. Demographic characteristics were reported, and COVID-19 RBD antibody levels examined in relation to time since primary vaccination. Linear regression was used to evaluate determinants of mean log anti-RBD antibody titers and compared by drug categories.
Results: A total of 352 participants completed a baseline visit and were eligible for analysis (Table 1). Participants were 75% women, 90% white, 20% Hispanic. Mean age: 57.4 (13.8), mean BMI 32.2 (9.4). The majority had RA (77%); 46% TNFi use, 28% JAKi, 27% others (abatacept, IL-17i) at primary vaccine series. Among those with no prior COVID-19 infection, anti-RBD IgG antibody titers showed a clear decrease with time since receipt of prior vaccination (Figure 1). In adjusted linear regression, referent to TNFi, JAKi (p=0.02) and abatacept (p=0.05) patients had lower mean titers, even after accounting for time since vaccine, number of vaccine doses and Anti-Nucleocapsid status. Table 2 shows the comparison of anti-Nucleocapsid status at baseline with participant self-report of prior COVID-19 infection, specificity was 78% (95% CI: 72%-83%) and sensitivity was 65% (55%-73%).
Conclusion: A clear decrease of anti-RBD IgG antibody levels in patients with RA or SpA taking immunomodulatory therapy was seen with increasing time following previous vaccine receipt. This effect was more pronounced in those who reported no prior COVID-19 infection, and who were receiving JAKi or abatacept (referent to TNFi). Self-report of prior COVID infection substantially misclassified prior COVID-19 infection and may confound immunogenicity outcomes in vaccine studies. Future analysis of the randomized trial results will look at the effect of holding or continuing immunomodulatory treatment at the time a SARS-CoV-2 booster is given.

.jpg)

Disclosures: A. Mudano, None; G. Cutter, AI Therapeutics, AMO Pharma., Astra-Zeneca,, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring,, Galmed Pharmaceuticals, Green Valley Pharma, Horizon Pharmaceuticals, mmunic, Mapi Pharmaceuticals LTD, Merck, Mitsubishi Tanabe Pharma Holdings, Opko Biologics, Prothena Biosciences, Novartis, Regeneron,, Sanofi-Aventis, Reata Pharmaceuticals, Alexion, Antisense Therapeutics, Biogen, Clinical Trial Solutions LLC, Genzyme, Genentech, GW Pharmaceuticals/Jazz, Klein-Buendel Incorporated, Merck/Serono, Osmotica Pharmaceuticals, Perception Neurosciences, Protalix Biotherapeutics,, Recursion/Cerexis Pharmaceuticals, Roche, SAB Biotherapeutics; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; G. Thiele, None; M. Law, None; B. Hamilton, None; M. Zikry, Labcorp, labcorp; K. Chun, None; M. Bastidas, LabCorp; M. George, AbbVie, GlaxoSmithKlein(GSK), Chemocentryx; L. Williams, None; K. Winthrop, AbbVie, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GSK, Pfizer, Roche, Regeneron, Sanofi, UCB, AstraZeneca, Novartis; M. Busch, Amgen, AbbVie/Abbott, GlaxoSmithKlein(GSK), UCB, horizon; S. Cohen, AbbVie/Abbott, Amgen, Gilead, Eli Lilly, Pfizer; R. Czubatyj, AbbVie/Abbott, Amgen, Horizon, Exagen, Janssen; R. Kataria, AbbVie/Abbott, Amgen, Horizon, Scipher; R. Khan, Amgen, Janssen, GlaxoSmithKlein(GSK); S. Mousa, None; J. Pando, AbbVie/Abbott, Novartis; E. Perkins, AbbVie/Abbott, Novartis, Janssen; S. Reddy, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Janssen, Novartis, Pfizer, UCB; D. Santos, Pfizer, Amgen, AbbVie/Abbott; J. Schechtman, Exagen, Mallinckrodt, Horizon, Pfizer, UCB, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Lilly, Myriad, Boehringer-Ingelheim, Genentech, Scipher, Janssen, Radius, Amgen, ChemoCentryx; F. Scott, None; S. Shanmugam, AstraZeneca; A. Singhal, None; J. Tesser, AbbVie, Amgen, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, GlaxoSmithKlein (GSK), Janssen, Gilead, Merck/MSD, Novartis, Pfizer, UCB, Aurinia, Genentech, Sanofi-Genzyme, Biogen, Celgene, CSL Behring, Horizon, R-Pharma, Regeneron, Roche, Sandoz, Scipher, Takeda, Sun Pharma, Selecta, Vorso; J. Tower, Amgen; S. Venuturupalli, Horizon Pharma USA, Inc., Kezar Life Sciences, Inc., Mallinckrodt, Inc., Navidea Biopharmaceuticals, Inc., Pfizer (Current), Bristol-Myers Squibb(BMS), Argenx, Janssen; S. Wollaston, None; C. Ziembinski, UCB, Scipher, CorEvitas; J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower.

